Biology:Steroid sulfatase
 Generic protein structure example |
| Steryl-sulfatase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 3.1.6.2 | ||||||||
| CAS number | 9025-62-1 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
Steroid sulfatase (STS), or steryl-sulfatase (EC 3.1.6.2), formerly known as arylsulfatase C, is a sulfatase enzyme involved in the metabolism of steroids. It is encoded by the STS gene.[1]
Reactions
This enzyme catalyses the following chemical reaction
- 3β-hydroxyandrost-5-en-17-one 3-sulfate + H2O [math]\displaystyle{ \rightleftharpoons }[/math] 3β-hydroxyandrost-5-en-17-one + sulfate
Also acts on some related steryl sulfates.
Function
The protein encoded by this gene catalyzes the conversion of sulfated steroid precursors to the free steroid. This includes DHEA sulfate, estrone sulfate, pregnenolone sulfate, and cholesterol sulfate, all to their unconjugated forms (DHEA, estrone, pregnenolone, and cholesterol, respectively).[2][3] The encoded protein is found in the endoplasmic reticulum, where it is present as a homodimer.[1]
Clinical significance
A congenital deficiency in the enzyme is associated with X-linked ichthyosis, a scaly-skin disease affecting roughly 1 in every 2,000 to 6,000 males.[5][6] The excessive skin scaling and hyperkeratosis is caused by a lack of breakdown and thus accumulation of cholesterol sulfate, a steroid that stabilizes cell membranes and adds cohesion, in the outer layers of the skin.[2]
Genetic deletions including STS are associated with an increased risk of developmental and mood disorders (and associated traits), and of atrial fibrillation or atrial flutter in males.[7] Both steroid sulfatase deficiency and common genetic risk variants within STS may confer increased atrial fibrillation risk.[8] Blood-clotting abnormalities may occur more frequently in males with XLI and female carriers.[9] Knockdown of STS gene expression in human skin cell cultures affects pathways associated with skin function, brain and heart development, and blood-clotting that may be relevant for explaining the skin condition and increased likelihood of ADHD/autism, cardiac arrhythmias and disorders of hemostasis in XLI.[10]
Steroid sulfates like DHEA sulfate and estrone sulfate serve as large biologically inert reservoirs for conversion into androgens and estrogens, respectively, and hence are of significance for androgen- and estrogen-dependent conditions like prostate cancer, breast cancer, endometriosis, and others. A number of clinical trials have been performed with inhibitors of the enzyme that have demonstrated clinical benefit, particularly in oncology and so far up to Phase II.[11] The non-steroidal drug Irosustat has been the most studied to date.
Inhibitors
Inhibitors of STS include irosustat, estrone sulfamate (EMATE), estradiol sulfamate (E2MATE), and danazol.[12][13] The most potent inhibitors are based around the aryl sulfamate pharmacophore[14] and it is thought that such compounds irreversibly modify the active site formylglycine residue of steroid sulfatase.[11]
Names
Steryl-sulfatase is also known as arylsulfatase, steroid sulfatase, sterol sulfatase, dehydroepiandrosterone sulfate sulfatase, arylsulfatase C, steroid 3-sulfatase, steroid sulfate sulfohydrolase, dehydroepiandrosterone sulfatase, pregnenolone sulfatase, phenolic steroid sulfatase, 3-beta-hydroxysteroid sulfate sulfatase, as well as by its systematic name steryl-sulfate sulfohydrolase.[15][16][17]
See also
- Steroidogenic enzyme
- Steroid sulfotransferase
- Estrogen sulfotransferase
References
- ↑ 1.0 1.1 "Entrez Gene: STS steroid sulfatase (microsomal), arylsulfatase C, isozyme S". https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=412.
- ↑ 2.0 2.1 "The Regulation of Steroid Action by Sulfation and Desulfation". Endocrine Reviews 36 (5): 526–63. October 2015. doi:10.1210/er.2015-1036. PMID 26213785.
- ↑ "The Important Roles of Steroid Sulfatase and Sulfotransferases in Gynecological Diseases". Frontiers in Pharmacology 7: 30. 2016. doi:10.3389/fphar.2016.00030. PMID 26924986.
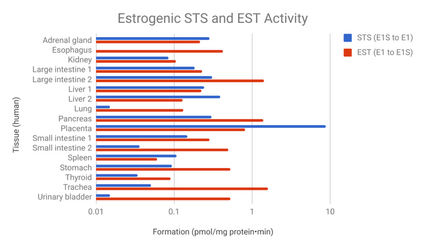
- ↑ "Systemic distribution of steroid sulfatase and estrogen sulfotransferase in human adult and fetal tissues". The Journal of Clinical Endocrinology and Metabolism 87 (12): 5760–8. December 2002. doi:10.1210/jc.2002-020670. PMID 12466383.
- ↑ "Characterization of point mutations in patients with X-linked ichthyosis. Effects on the structure and function of the steroid sulfatase protein". The Journal of Biological Chemistry 272 (33): 20756–63. August 1997. doi:10.1074/jbc.272.33.20756. PMID 9252398.
- ↑ "Mutations in X-linked ichthyosis disrupt the active site structure of estrone/DHEA sulfatase". Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1739 (1): 1–4. December 2004. doi:10.1016/j.bbadis.2004.09.003. PMID 15607112.
- ↑ "Medical and neurobehavioural phenotypes in carriers of X-linked ichthyosis-associated genetic deletions in the UK Biobank". Journal of Medical Genetics 57 (10): 692–698. Mar 2020. doi:10.1136/jmedgenet-2019-106676. PMID 32139392.
- ↑ "Characterising heart rhythm abnormalities associated with Xp22.31 deletion". Journal of Medical Genetics 60 (7): 636–643. November 2022. doi:10.1136/jmg-2022-108862. PMID 36379544.
- ↑ Brcic L, Wren GH, Underwood JFG, Kirov G, Davies W (2022) Comorbid medical issues in X-linked ichthyosis. JID Innovations 2(3):100109 PMID 35330591 doi:10.1016/j.xjidi.2022.100109 URL: https://www.jidinnovations.org/article/S2667-0267(22)00016-9/fulltext
- ↑ McGeoghan F, Camera E, Maiellaro M, Menon M, Huang M, Dewan P, Ziaj S, Caley MP, Donaldson M, Enright AJ, O'Toole EA (2023) RNA sequencing and lipidomics uncovers novel pathomechanisms in recessive X-linked ichthyosis Frontiers in Molecular Biosciences 10:1176802 PMID 37363400 doi:10.3389/fmolb.2023.1176802 URL:https://www.frontiersin.org/articles/10.3389/fmolb.2023.1176802/full
- ↑ 11.0 11.1 "SULFATION PATHWAYS: Steroid sulphatase inhibition via aryl sulphamates: clinical progress, mechanism and future prospects". Journal of Molecular Endocrinology 61 (2): T233–T252. August 2018. doi:10.1530/JME-18-0045. PMID 29618488. https://jme.bioscientifica.com/view/journals/jme/61/2/JME-18-0045.xml.
- ↑ "Estrogen O-sulfamates and their analogues: Clinical steroid sulfatase inhibitors with broad potential". The Journal of Steroid Biochemistry and Molecular Biology 153: 160–9. September 2015. doi:10.1016/j.jsbmb.2015.03.012. PMID 25843211.
- ↑ "Inhibition of steroid sulfatase activity by danazol". Acta Obstetricia et Gynecologica Scandinavica Supplement 123: 107–11. 1984. doi:10.3109/00016348409156994. PMID 6238495.
- ↑ "Discovery and Development of the Aryl O-Sulfamate Pharmacophore for Oncology and Women's Health". Journal of Medicinal Chemistry 58 (19): 7634–58. October 2015. doi:10.1021/acs.jmedchem.5b00386. PMID 25992880.
- ↑ "The steroid sulphatase of Patella vulgata". Biochimica et Biophysica Acta 15 (2): 300–1. October 1954. doi:10.1016/0006-3002(54)90078-5. PMID 13208702.
- ↑ "The Synthesis and Hydrolysis of Sulfate Esters". Advances in Enzymology and Related Areas of Molecular Biology. Advances in Enzymology - and Related Areas of Molecular Biology. 22. 1960. 205–35. doi:10.1002/9780470122679.ch5. ISBN 9780470122679.
- ↑ "The enzymic hydrolysis of steroid conjugates. I. Sulphatase and β-glucuronidase activity of molluscan extracts". The Biochemical Journal 63 (4): 705–10. August 1956. doi:10.1042/bj0630705. PMID 13355874.
Further reading
- "Basis for abnormal desquamation and permeability barrier dysfunction in RXLI". The Journal of Investigative Dermatology 122 (2): 314–9. February 2004. doi:10.1046/j.1523-1747.2003.22258.x. PMID 15009711.
- "Identification of point mutations in the steroid sulfatase gene of three patients with X-linked ichthyosis". American Journal of Human Genetics 50 (3): 483–91. March 1992. PMID 1539590.
- "Characterization of arylsulfatase C isozymes from human liver and placenta". Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology 1078 (2): 251–7. June 1991. doi:10.1016/0167-4838(91)90566-I. PMID 2065092.
- "Cloning and expression of human steroid-sulfatase. Membrane topology, glycosylation, and subcellular distribution in BHK-21 cells". The Journal of Biological Chemistry 264 (23): 13865–72. August 1989. doi:10.1016/S0021-9258(18)80080-1. PMID 2668275.
- "Characterization of rat and human steroid sulfatases". Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology 997 (3): 199–205. August 1989. doi:10.1016/0167-4838(89)90187-8. PMID 2765556.
- "Cloning and expression of steroid sulfatase cDNA and the frequent occurrence of deletions in STS deficiency: implications for X-Y interchange". Cell 49 (4): 443–54. May 1987. doi:10.1016/0092-8674(87)90447-8. PMID 3032454.
- "Genetic heterogeneity of steroid sulfatase deficiency revealed with cDNA for human steroid sulfatase". Biochemical and Biophysical Research Communications 144 (2): 1010–7. April 1987. doi:10.1016/S0006-291X(87)80064-5. PMID 3034252.
- "X/Y translocation in a family with X-linked ichthyosis, chondrodysplasia punctata, and mental retardation: DNA analysis reveals deletion of the steroid sulphatase gene and translocation of its Y pseudogene". Clinical Genetics 34 (1): 31–7. July 1988. doi:10.1111/j.1399-0004.1988.tb02612.x. PMID 3165728.
- "The human X-linked steroid sulfatase gene and a Y-encoded pseudogene: evidence for an inversion of the Y chromosome during primate evolution". Cell 55 (6): 1123–35. December 1988. doi:10.1016/0092-8674(88)90257-7. PMID 3203382.
- "Association of steroid sulfatase with one of the arylsulfatase C isozymes in human fibroblasts". The Journal of Biological Chemistry 261 (31): 14443–7. November 1986. doi:10.1016/S0021-9258(18)66889-9. PMID 3464600.
- "Tissue-specific expression of human arylsulfatase-C isozymes and steroid sulfatase". American Journal of Human Genetics 40 (2): 102–14. February 1987. PMID 3471087.
- "Further evidence for the assignment of the steroid sulfatase X-linked ichthyosis locus to the telomer of Xp". Human Genetics 58 (4): 446–447. 1982. doi:10.1007/bf00282842. PMID 6948769.
- "Differential expression of steroid sulphatase locus on active and inactive human X chromosome". Nature 299 (5886): 838–40. October 1982. doi:10.1038/299838a0. PMID 6957717. Bibcode: 1982Natur.299..838M.
- "Normalization and subtraction: two approaches to facilitate gene discovery". Genome Research 6 (9): 791–806. September 1996. doi:10.1101/gr.6.9.791. PMID 8889548.
- "Characterization of point mutations in patients with X-linked ichthyosis. Effects on the structure and function of the steroid sulfatase protein". The Journal of Biological Chemistry 272 (33): 20756–63. August 1997. doi:10.1074/jbc.272.33.20756. PMID 9252398.
- "PCR diagnosis of X-linked ichthyosis: identification of a novel mutation (E560P) of the steroid sulfatase gene". Human Mutation 15 (3): 296. March 2000. doi:10.1002/(SICI)1098-1004(200003)15:3<296::AID-HUMU17>3.0.CO;2-#. PMID 10679952.
- "Novel point mutations in the steroid sulfatase gene in patients with X-linked ichthyosis: transfection analysis using the mutated genes". The Journal of Investigative Dermatology 114 (6): 1195–9. June 2000. doi:10.1046/j.1523-1747.2000.00004.x. PMID 10844566.
- "Deletion pattern of the STS gene in X-linked ichthyosis in a Mexican population". Molecular Medicine 7 (12): 845–9. December 2001. doi:10.1007/BF03401976. PMID 11844872.
- "Steroid sulfatase in the human hair follicle concentrates in the dermal papilla". The Journal of Investigative Dermatology 117 (6): 1342–8. December 2001. doi:10.1046/j.0022-202x.2001.01547.x. PMID 11886493.
- "Regulation of estrogen activity in human endometrium: effect of IL-1β on steroid sulfatase activity in human endometrial stromal cells". Steroids 67 (7): 655–9. June 2002. doi:10.1016/S0039-128X(02)00016-8. PMID 11996939.
External links
- Steryl-Sulfatase at the US National Library of Medicine Medical Subject Headings (MeSH)
 |