Chemistry:2-Methylbenzaldehyde
From HandWiki
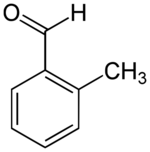
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methylbenzaldehyde | |
| Other names
o-Tolualdehyde
| |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| 605841 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| 3304 | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H8O | |
| Molar mass | 120.151 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.0328 g/cm3 (20 °C) |
| Melting point | −35 °C (−31 °F; 238 K) |
| Boiling point | 199–200 °C (390–392 °F; 472–473 K) |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H302, H314, H315, H319, H335 | |
| P260, P261, P264, P270, P271, P280, P301+312, P301+330+331, P302+352, P303+361+353, P304+340, P305+351+338, P310, P312, P321, P330, P332+313, P337+313, P362, P363, P403+233, P405, P501 | |
| Flash point | 67 °C; 153 °F; 340 K |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
2-Methylbenzaldehyde is an organic compound with the formula CH3C6H4CHO. It is a colorless liquid.[1]
Use and occurrence
Of its many reactions, 2-methylbenzaldehyde undergoes BF3-induced Rothemund condensation with pyrrole to give atropoisomers of tetrakis(o-tolyl)porphyrin.[2]
It is one of main benzaldehyde component of automobile exhaust.[3]
Related compounds
References
- ↑ H. B. Hass; Myron L. Bender (1950). "o-Tolualdehyde". Org. Synth. 30: 99. doi:10.15227/orgsyn.030.0099.
- ↑ Lindsey, Jonathan S.; Wagner, Richard W. (1989). "Investigation of the synthesis of ortho-substituted tetraphenylporphyrins". J. Org. Chem. 54 (4): 828–36. doi:10.1021/jo00265a021.
- ↑ Rogge, Wolfgang F.; Hildemann, Lynn M.; Mazurek, Monica A.; Cass, Glen R.; Simoneit, Bernd R. T. (1993). "Sources of fine organic aerosol. 2. Noncatalyst and catalyst-equipped automobiles and heavy-duty diesel trucks". Environmental Science and Technology 27 (4): 636–51. doi:10.1021/es00041a007.
 |

