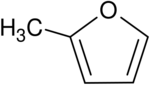
Chemistry:2-Methylfuran
From HandWiki
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methylfuran | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C5H6O | |
| Molar mass | 82.10 g/mol |
| Appearance | Colorless to pale yellow/green liquid |
| Density | 0.91546 g/mL (20 °C) [1] |
| Boiling point | 64[2][1] °C (147 °F; 337 K) |
| 3000 mg/L (20 °C) | |
| Solubility in ethanol | Soluble |
Refractive index (nD)
|
1.4332 (20 °C) [1] |
| Hazards | |
| Main hazards | Very flammable, harmful |
| NFPA 704 (fire diamond) | |
| Flash point | −22 °C; −8 °F; 251 K |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
2-Methylfuran, also known with the older name of sylvane, is a flammable, water-insoluble liquid[3] with a chocolate odor, found naturally in Myrtle and Dutch Lavender[4] used as a FEMA GRAS flavoring substance,[5] with the potential for use in alternative fuels.
Manufacture
2-Methylfuran is an article of commerce (chemical intermediate) and is normally manufactured by catalytic hydrogenolysis of furfural alcohol or via a hydrogenation-hydrogenolysis sequence from furfural in the vapor phase.[6]
See also
- Swiftfuel
References
- ↑ 1.0 1.1 1.2 Baird, Zachariah Steven; Uusi-Kyyny, Petri; Pokki, Juha-Pekka; Pedegert, Emilie; Alopaeus, Ville (6 Nov 2019). "Vapor Pressures, Densities, and PC-SAFT Parameters for 11 Bio-compounds". International Journal of Thermophysics 40 (11): 102. doi:10.1007/s10765-019-2570-9. Bibcode: 2019IJT....40..102B.
- ↑ NIST Chemistry WebBook. http://webbook.nist.gov
- ↑ Kenneth Barbalace. "Chemical Database - 2-Methylfuran. EnvironmentalChemistry.com. 1995 - 2008. Accessed on-line: 8/26/2008". http://environmentalchemistry.com/yogi/chemicals/cn/2-Methylfuran.html. Retrieved 2008-08-26.
- ↑ Jim Duke. "Dr. Duke's Phytochemical and Ethnobotanical Databases. [Online Database 26 August 2008. 2-METHYL-FURAN"]. https://phytochem.nal.usda.gov/phytochem/search/list. Retrieved 2008-08-26.
- ↑ "2-methyl furan". The Good Scents Company.. http://www.thegoodscentscompany.com/data/rw1023171.html. Retrieved 2008-08-26.
- ↑ L. W. Burnette, et al., "Production of 2-Methylfuran by Vapor Phase Hydrogenation of Furfural" Industrial and engineering Chemistry, V40, P502-505 (1948).
External links
 |


