Chemistry:Furfural
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Furan-2-carbaldehyde | |||
| Other names
Furfural, furan-2-carboxaldehyde, fural, furfuraldehyde, 2-furaldehyde, pyromucic aldehyde
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C5H4O2 | |||
| Molar mass | 96.085 g·mol−1 | ||
| Appearance | Colorless oil | ||
| Odor | Almond-like[1] | ||
| Density | 1.1601 g/mL (20 °C)[2][3] | ||
| Melting point | −37 °C (−35 °F; 236 K)[2] | ||
| Boiling point | 162 °C (324 °F; 435 K)[2] | ||
| 83 g/L[2] | |||
| Vapor pressure | 2 mmHg (20 °C)[1] | ||
| −47.1×10−6 cm3/mol | |||
| Hazards | |||
| Flash point | 62 °C (144 °F; 335 K) | ||
| Explosive limits | 2.1–19.3%[1] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
300–500 mg/kg (oral, mice)[4] | ||
LC50 (median concentration)
|
| ||
LCLo (lowest published)
|
| ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 5 ppm (20 mg/m3) [skin][1] | ||
REL (Recommended)
|
No established REL[1] | ||
IDLH (Immediate danger)
|
100 ppm[1] | ||
| Related compounds | |||
Related Furan-2-carbaldehydes
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
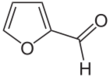
Furfural is an organic compound with the formula C4H3OCHO. It is a colorless liquid, although commercial samples are often brown. It has an aldehyde group attached to the 2-position of furan. It is a product of the dehydration of sugars, as occurs in a variety of agricultural byproducts, including corncobs, oat, wheat bran, and sawdust. The name furfural comes from the Latin word furfur, meaning bran, referring to its usual source. Furfural is only derived from dryed biomass, In addition to ethanol, acetic acid, and sugar, furfural is one of the oldest organic chemicals available readily purified from natural precursors.[6]
History
Furfural was first isolated in 1821 (published in 1832) by the German chemist Johann Wolfgang Döbereiner, who produced a small sample as a byproduct of formic acid synthesis.[7] In 1840, the Scottish chemist John Stenhouse found that the same chemical could be produced by distilling a wide variety of crop materials, including corn, oats, bran, and sawdust, with aqueous sulfuric acid; he also determined furfural's empirical formula (C5H4O2).[8] George Fownes named this oil "furfurol" in 1845 (from furfur (bran), and oleum (oil)).[9] In 1848, the French chemist Auguste Cahours determined that furfural was an aldehyde.[10] Determining the structure of furfural required some time: the furfural molecule contains a cyclic ether (furan), which tends to break open when it's treated with harsh reagents. In 1870, German chemist Adolf von Baeyer speculated about the structure of the chemically similar compounds furan and 2-furoic acid.[11] Additional research by German chemist Heinrich Limpricht supported this idea.[12] From work published in 1877, Baeyer had confirmed his previous belief on the structure of furfural.[13] By 1886, furfurol was being called "furfural" (short for "furfuraldehyde") and the correct chemical structure for furfural was being proposed.[14] By 1887, the German chemist Willy Marckwald had inferred that some derivatives of furfural contained a furan nucleus.[15] In 1901, the German chemist Carl Harries determined furan's structure through work with succindialdehyde and 2-methylfuran, thereby also confirming furfural's proposed structure.[16][17]
Furfural remained relatively obscure until 1922,[6] when the Quaker Oats Company began mass-producing it from oat hulls.[18] Today, furfural is still produced from agricultural byproducts like sugarcane bagasse and corn cobs. The main countries producing furfural today are the Dominican Republic, South Africa and China.
Properties
Furfural dissolves readily in most polar organic solvents, but it is only slightly soluble in either water or alkanes.
Furfural participates in the same kinds of reactions as other aldehydes and other aromatic compounds. It exhibits less aromatic character than benzene, as can be seen from the fact that furfural is readily hydrogenated to tetrahydrofurfuryl alcohol. When heated in the presence of acids, furfural irreversibly polymerizes, acting as a thermosetting polymer.
Production
Furfural may be obtained by the acid catalyzed dehydration of 5-carbon sugars (pentoses), particularly xylose.[19]
- C5H10O5 → C5H4O2 + 3 H2O
These sugars may be obtained from pentosans obtained from hemicellulose present in lignocellulosic biomass.
Between 3% and 10% of the mass of crop residue feedstocks can be recovered as furfural, depending on the type of feedstock. Furfural and water evaporate together from the reaction mixture, and separate upon condensation. The global production capacity is about 800,000 tons as of 2012. China is the biggest supplier of furfural, and accounts for the greater part of global capacity. The other two major commercial producers are Illovo Sugar in South Africa and Central Romana in the Dominican Republic.[20]
In the laboratory, furfural can be synthesized from plant material by heating with sulfuric acid[21] or other acids.[22][20] With the purpose to avoid toxic effluents, an effort to substitute sulfuric acid with easily separable and reusable solid acid catalysts has been studied around the world.[23] The formation and extraction of xylose and subsequently furfural can be favored over the extraction of other sugars with varied conditions, such as acid concentration, temperature, and time.
In industrial production, some lignocellulosic residue remains after the removal of the furfural.[24] This residue is dried and burned to provide steam for the operation of the furfural plant. Newer and more energy efficient plants have excess residue, which is or can be used for co-generation of electricity,[25][26] cattle feed, activated carbon, mulch/fertiliser, etc.
Uses and occurrence
It is commonly found in many cooked or heated foods such as coffee (55–255 mg/kg) and whole grain bread (26 mg/kg).[4]
Furfural is an important renewable, non-petroleum based, chemical feedstock. It can be converted into a variety of solvents, polymers, fuels and other useful chemicals by a range of catalytic reduction.[27]
Hydrogenation of furfural provides furfuryl alcohol (FA), which is used to produce furan resins, which are exploited in thermoset polymer matrix composites, cements, adhesives, casting resins and coatings.[28] Further hydrogenation of furfuryl alcohol leads to tetrahydrofurfuryl alcohol (THFA), which is used as a solvent in agricultural formulations and as an adjuvant to help herbicides penetrate the leaf structure.
In another application as a feedstock, palladium-catalyzed decarbonylation on furfural manufactures industrially furan.[4]
Another important solvent made from furfural is methyltetrahydrofuran. Furfural is used to make other furan derivatives, such as furoic acid, via oxidation,[29] and furan itself via palladium catalyzed vapor phase decarbonylation.[4]
Furfural is also a specialized chemical solvent.[20]
There is a good market for value added chemicals that can be obtained from furfural.[20]
Safety
Furfural is carcinogenic in lab animals and mutagenic in single cell organisms, but there is no data on human subjects. It is classified in IARC Group 3 due to the lack of data on humans and too few tests on animals to satisfy Group 2A/2B criteria. It is hepatotoxic.[30][31][32][33]
The median lethal dose is high, 650–900 mg/kg (oral, dogs), consistent with its pervasiveness in foods.[4]
The Occupational Safety and Health Administration has set a permissible exposure limit for furfural at 5 ppm over an eight-hour time-weighted average (TWA), and also designates furfural as a risk for skin absorption.[1]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 NIOSH Pocket Guide to Chemical Hazards. "#0297". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0297.html.
- ↑ 2.0 2.1 2.2 2.3 Record of CAS RN 98-01-1 in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ↑ Baird, Zachariah Steven; Uusi-Kyyny, Petri; Pokki, Juha-Pekka et al. (6 Nov 2019). "Vapor Pressures, Densities, and PC-SAFT Parameters for 11 Bio-compounds". International Journal of Thermophysics 40 (11): 102. doi:10.1007/s10765-019-2570-9. Bibcode: 2019IJT....40..102B.
- ↑ 4.0 4.1 4.2 4.3 4.4 Hoydonckx, H. E.; Van Rhijn, W. M.; Van Rhijn, W.; De Vos, D. E.; Jacobs, P. A.. "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a12_119.pub2.
- ↑ 5.0 5.1 "Furfural". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/98011.html.
- ↑ 6.0 6.1 Peters, Fredus N. (1936). "The Furans: Fifteen Years of Progress". Industrial & Engineering Chemistry 28 (7): 755–759. doi:10.1021/ie50319a002. ISSN 0019-7866.
- ↑ J. W. Döbereiner (1832). "Ueber die medicinische und chemische Anwendung und die vortheilhafte Darstellung der Ameisensäure" (in de). Annalen der Pharmacie 3 (2): 141–146. doi:10.1002/jlac.18320030206. https://zenodo.org/record/1426896. From p. 141: "Ich verbinde mit diese Bitte noch die Bemerkung, … Bittermandelöl riechende Materie enthält, … " (I join to this request also the observation that the formic acid which is formed by the simultaneous reaction of sulfuric acid and manganese peroxide with sugar and which contains a volatile material that appears oily in an isolated condition and that smells like a mixture of cassia and bitter almond oil … )
- ↑ John Stenhouse (1843). "On the Oils Produced by the Action of Sulphuric Acid upon Various Classes of Vegetables. [Abstract"]. Abstracts of the Papers Communicated to the Royal Society of London 5: 939–941. doi:10.1098/rspl.1843.0234. https://zenodo.org/record/1432029.
- See also: Stenhouse, John (1850). "On the oils produced by the action of sulphuric acid upon various classes of vegetables". Philosophical Transactions of the Royal Society of London 140: 467–480. doi:10.1098/rstl.1850.0024.
- ↑ George Fownes (1845). "An Account of the Artificial Formation of a Vegeto-Alkali". Philosophical Transactions of the Royal Society of London 135: 253–262. doi:10.1098/rstl.1845.0008.
- ↑ Cahours, Auguste (1848). "Note sur le furfurol" (in fr). Annales de Chimie et de Physique. 3rd series 24: 277–285. https://babel.hathitrust.org/cgi/pt?id=uc1.$b617955&view=1up&seq=285. (English translation: Cahours, A. (1848). "Observations on furfurol". The Chemical Gazette 6: 457–460. https://books.google.com/books?id=lwMAAAAAMAAJ&pg=PA457.)
- ↑ Baeyer, A.; Emmerling, A. (1870). "Reduction des Istatins zu Indigblau" (in de). Berichte der Deutschen Chemischen Gesellschaft 3: 514–517. doi:10.1002/cber.187000301169. https://babel.hathitrust.org/cgi/pt?id=uc1.b3481748&view=1up&seq=520.
- ↑ Limpricht, H. (1870). "Ueber das Tetraphenol C4H4O" (in de). Berichte der Deutschen Chemischen Gesellschaft 3: 90–91. doi:10.1002/cber.18700030129. https://babel.hathitrust.org/cgi/pt?id=uc1.b3481748&view=1up&seq=96. From p. 90: "Die Ansicht, dass die Pyroschleimsäure eine der Salicylsäure ähnliche Constitution besitzt, macht das Auftreten des Tetraphenols bei der Destillation der pyroschleimsauren Salze wahrscheinlich." (The belief that 2-furoic acid has a structure similar to salicylic acid makes probable the presence of tetraphenol [furan] during the distillation of salts of 2-furoic acid.) That is, just as heating salts of salicylic acid produces phenol, so heating salts of 2-furoic acid should produce an analog of phenol containing 4 carbon atoms.
- ↑ In 1877, Baeyer published a series of papers on furfural, as he tried to determine its structure.
- Baeyer, Adolf (1877). "Ueber das Furfurol. Erste Mittheilung." (in de). Berichte der Deutschen Chemischen Gesellschaft 10: 355–358. doi:10.1002/cber.187701001101. https://babel.hathitrust.org/cgi/pt?id=hvd.cl1hz2&view=1up&seq=385.
- Baeyer, Adolf (1877). "Ueber das Furfurol. Zweite Mittheilung." (in de). Berichte der Deutschen Chemischen Gesellschaft 10: 695–698. doi:10.1002/cber.187701001195. https://babel.hathitrust.org/cgi/pt?id=hvd.cl1hz2&view=1up&seq=729.
- Baeyer, Adolf (1877). "Ueber das Furfurol. III. Mittheilung." (in de). Berichte der Deutschen Chemischen Gesellschaft 10 (2): 1358–1364. doi:10.1002/cber.18770100225. https://babel.hathitrust.org/cgi/pt?id=hvd.cl1hz3&view=1up&seq=86.
- ↑ Tilden, William A., ed (1886). Watts' Manual of Chemistry: Theoretical and Practical. II: Chemistry of Carbon-Compounds or, Organic Chemistry (2nd ed.). Philadelphia, Pennsylvania, USA: P. Blakiston, Son, & Co.. pp. 379–380. https://books.google.com/books?id=uoRNAAAAYAAJ&pg=PA379.
- ↑ Marckwald, W. (1887). "Zur Kenntnis der Furfuranverbindungen" (in de). Berichte der Deutschen Chemischen Gesellschaft 20 (2): 2811–2817. doi:10.1002/cber.188702002140. https://babel.hathitrust.org/cgi/pt?id=hvd.cl1hzr&view=1up&seq=561.
- ↑ Harries, C. (1901). "Ueber den Succindialdehyd" (in de). Berichte der Deutschen Chemischen Gesellschaft 34 (2): 1488–1498. doi:10.1002/cber.19010340225. https://babel.hathitrust.org/cgi/pt?id=iau.31858002459323&view=1up&seq=152.
- ↑ Harries, C. (1898). "Ueber die Aufspaltung des Sylvans zum Aldehyd der Lävulinsäure, Pentanonal. Untersuchungen über Bestandtheile des Buchentheers. I." (in de). Berichte der Deutschen Chemischen Gesellschaft 31 (1): 37–47. doi:10.1002/cber.18980310109. https://babel.hathitrust.org/cgi/pt?id=iau.31858002475550&view=1up&seq=47.
- ↑ Brownlee, Harold J.; Miner, Carl S. (1948). "Industrial Development of Furfural". Industrial & Engineering Chemistry 40 (2): 201–204. doi:10.1021/ie50458a005. ISSN 0019-7866.
- ↑ Cai, Charles M.; Zhang, Taiying; Kumar, Rajeev; Wyman, Charles E. (2014). "Integrated furfural production as a renewable fuel and chemical platform from lignocellulosic biomass". Journal of Chemical Technology & Biotechnology 89: 2–10. doi:10.1002/jctb.4168.
- ↑ 20.0 20.1 20.2 20.3 Dalvand, Kaveh (2018). "Economics of biofuels: Market potential of furfural and its derivatives". Biomass and Bioenergy 115: 56–63. doi:10.1016/j.biombioe.2018.04.005.
- ↑ Adams, Roger; Voorhees, V. (1921). "Furfural". Organic Syntheses 1: 49. doi:10.15227/orgsyn.001.0049.
- ↑ Zeitsch, Karl J. (2000). The chemistry and technology of furfural and its many by-products. Amsterdam: Elsevier. ISBN 978-0-08-052899-1. OCLC 162130560. https://archive.org/details/chemistrytechnol00kjze.
- ↑ Gómez Millán, Gerardo; El Assal, Zouhair; Nieminen, Kaarlo et al. (15 December 2018). "Fast furfural formation from xylose using solid acid catalysts assisted by a microwave reactor". Fuel Processing Technology 182: 56–67. doi:10.1016/j.fuproc.2018.10.013.
- ↑ Gómez Millán, Gerardo; Bangalore Ashok, R.P.; Oinas, Pekka et al. (8 April 2020). "Furfural production from xylose and birch hydrolysate liquor in a biphasic system and techno-economic analysis". Biomass Conversion and Biorefinery 11 (5): 2095–2106. doi:10.1007/s13399-020-00702-4.
- ↑ Edgard, Gnansounou, ed (2016-12-20). Life-cycle assessment of biorefineries. Amsterdam, Netherlands: Elsevier. ISBN 978-0-444-63586-0. OCLC 967224456.
- ↑ Virtual biorefinery: an optimization strategy for renewable carbon valorization. Cham: Springer. 2015-12-09. ISBN 978-3-319-26045-7. OCLC 932064033.
- ↑ Chen, Shuo; Wojcieszak, Robert; Dumeignil, Franck et al. (26 October 2018). "How Catalysts and Experimental Conditions Determine the Selective Hydroconversion of Furfural and 5-Hydroxymethylfurfural". Chemical Reviews 118 (22): 11023–11117. doi:10.1021/acs.chemrev.8b00134. PMID 30362725.
- ↑ Brydson, J. A. (1999). "Furan Resins". in J. A. Brydson. Plastics Materials (Seventh ed.). Oxford: Butterworth-Heinemann. pp. 810–813. doi:10.1016/B978-075064132-6/50069-3. ISBN 978-0-7506-4132-6. https://archive.org/details/plasticsmaterial00bryd.
- ↑ R. J. Harrison; M. Moyle (1956). "2-Furoic Acid". Organic Syntheses 36: 36. doi:10.15227/orgsyn.036.0036.
- ↑ "Furfural (CAS 98-01-1)". https://toxnet.nlm.nih.gov/cpdb/chempages/FURFURAL.html.
- ↑ "Dry Cleaning, Some Chlorinated Solvents and Other Industrial Chemicals". IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 63: 393–407. 1995. PMID 9097102. PMC 7681282. https://monographs.iarc.fr/wp-content/uploads/2018/06/mono63.pdf. Retrieved 24 November 2018.
- ↑ "Furfural(Group 3)". http://www.inchem.org/documents/iarc/vol63/furfural.html.
- ↑ Richard Irwin, Ph.D. (1990). NTP TECHNICAL REPORT ON THE TOXICOLOGY AND CARCINOGENESIS STUDIES OF FURFURAL (CAS NO. 98-01-1) IN F344/N RATS AND B6C3F1 MICE (GAVAGE STUDIES) (Report). U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES. https://ntp.niehs.nih.gov/ntp/htdocs/lt_rpts/tr382.pdf. Retrieved 24 November 2018.
External links
- Furfural in the Pesticide Properties DataBase (PPDB)
 |