Chemistry:3,4-Dimethoxystyrene
From HandWiki
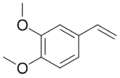
| |
| Names | |
|---|---|
| Preferred IUPAC name
4-Ethenyl-1,2-dimethoxybenzene | |
| Other names
1,2-Dimethoxy-4-vinylbenzene
4-Vinylveratrole 4-Vinyl-1,2-dimethoxybenzene | |
| Identifiers | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| Properties | |
| C10H12O2 | |
| Molar mass | 164.204 g·mol−1 |
| Appearance | Yellowish oily liquid |
| Odor | Sweet, floral |
| Density | 1.109 g/cm3 |
| Boiling point | 110–125 °C (230–257 °F; 383–398 K) |
Refractive index (nD)
|
1.571 |
| Hazards | |
| Main hazards | flammable, toxic |
| Safety data sheet | MSDS |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H319 | |
| P264, P280, P305+351+338, P337+313 | |
| Related compounds | |
Related styrenes;
related aromatic compounds |
styrene, dimethoxybenzene |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
3,4-Dimethoxystyrene (vinylveratrole) is an aromatic organic compound. It is a yellow oily liquid with a pleasant floral odor. Normally, it is supplied with 1-2% of the hydroquinone as an additive to prevent oxidation of the compound.
Occurrence
3,4-Dimethoxystyrene is found in the essential oil of Brazilian propolis.[1]
Uses
- 3,4-Dimethoxystyrene is typically used in organic synthesis as a monomer in radical polymerization reactions due to the presence of the electron-deficient double bond.[2]
- 3,4-dimethoxystyrene can be deprotected using Lewis acid boron tribromide with almost 100% yield. The resulting compound 3,4-dihydroxystyrene is rapidly oxidized in air this is why 3,4-dimethoxystyrene is preferred as a stable precursor in organic synthesis.
- To the chemical community, it presents interest as an easily polymerizable precursor to polycatechols as it is less susceptible to oxidation in air.[3]
Related compounds
- 3,4-Dihydroxystyrene
- Veratrole
References
- ↑ Kusumoto, Toshihide; Tomofumi, Miyamoto; Ryuichi, Higuchi; Shima, Doi; Hiroyuki, Sugimoto; Hideo, Yamada (2001). "Isolation and Structures of Two New Compounds from the Essential Oil of Brazilian Propolis". Chem. Pharm. Bull. 49 (9): 1207–1209. doi:10.1248/cpb.49.1207. PMID 11558615. http://cpb.pharm.or.jp/cpb/200109/c09_1207.pdf. Retrieved 10 September 2015.
- ↑ Rooney, J.M. (1983). "Cationic polymerization of 3,4-dimethoxystyrene by trityl hexachloroantimonate". Polymer Bulletin 10 (9–10): 414–418. doi:10.1007/bf00262183.
- ↑ Daly, William H.; Moulay, Saad (2007). "Synthesis of poly (vinylcatechols)". Journal of Polymer Science: Polymer Symposia 74: 227–242. doi:10.1002/polc.5070740120. https://digitalcommons.lsu.edu/gradschool_disstheses/4197.
 |
