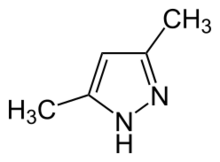
Chemistry:3,5-Dimethylpyrazole
From HandWiki

| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
3,5-Dimethyl-1H-pyrazole | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C5H8N2 | |
| Molar mass | 96.133 g·mol−1 |
| Appearance | white solid |
| Density | 1.027 g/cm3 |
| Melting point | 107.5 °C (225.5 °F; 380.6 K) |
| Boiling point | 218 °C (424 °F; 491 K) |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H302, H315, H319, H335, H361, H373 | |
| P201, P202, P260, P261, P264, P270, P271, P280, P281, P301+312, P302+352, P304+340, P305+351+338, P308+313, P312, P314, P321, P330, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
3,5-Dimethylpyrazole is an organic compound with the formula (CH3C)2CHN2H. It is one of several isomeric derivatives of pyrazole that contain two methyl substituents. The compound is unsymmetrical but the corresponding conjugate acid (pyrazolium) and conjugate base (pyrazolide) have C2v symmetry. It is a white solid that dissolves well in polar organic solvents.
It is a precursor to a variety of ligands that are widely studied in coordination chemistry including trispyrazolylborate, a trispyrazolylmethane, and a pyrazolyldiphosphine.[1][2]
Condensation of acetylacetone and hydrazine gives 3,5-dimethylpyrazole:[3]
- CH3C(O)CH2C(O)CH3 + N2H4 → (CH3C)2CHN2H + 2 H2O
It has found use as a blocking agent for isocyanates.[4]
References
- ↑ Reger, Daniel L.; Grattan, T.Christian; Brown, Kenneth J.; Little, Christine A.; Lamba, Jaydeep J.S.; Rheingold, Arnold L.; Sommer, Roger D. (2000). "Syntheses of tris(pyrazolyl)methane ligands and {[tris(pyrazolyl)methane]Mn(CO)3}SO3CF3 complexes: Comparison of ligand donor properties". Journal of Organometallic Chemistry 607 (1–2): 120–128. doi:10.1016/S0022-328X(00)00290-4.
- ↑ Schenck, Terry G.; Downes, J. M.; Milne, C. R. C.; MacKenzie, Peter B.; Boucher, Terry G.; Whelan, John; Bosnich, B. (1985). "Bimetallic reactivity. Synthesis of bimetallic complexes containing a bis(phosphino)pyrazole ligand". Inorganic Chemistry 24 (15): 2334–2337. doi:10.1021/ic00209a003.
- ↑ Johnson, William S.; Highet, Robert J. (1951). "3,5-Dimethylpyrazole". Organic Syntheses 31: 43. doi:10.15227/orgsyn.031.0043.
- ↑ "Blocked Isocyanates". June 2020. https://ure.lanxess.com/wp-content/uploads/sites/26/2020/07/Blocked-Isocyanates.pdf.
 |

