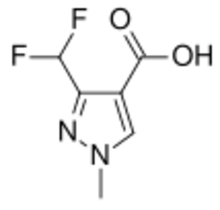
Chemistry:3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid
| |
| Names | |
|---|---|
| Preferred IUPAC name
3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid | |
| Other names
CSAA798670, NOA449410
| |
| Identifiers | |
3D model (JSmol)
|
|
| 11698637 | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties[1] | |
| C6H6F2N2O2 | |
| Molar mass | 176.12 |
| Melting point | 200–201°C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid is a chemical compound which is used commercially as an intermediate to seven fungicides which act by inhibition of succinate dehydrogenase (SDHI).[2] It consists of a pyrazole ring with difluoromethyl, methyl and carboxylic acid groups attached in specific positions.
Background
Inhibition of succinate dehydrogenase, the complex II in the mitochondrial respiration chain, has been known as a fungicidal mechanism of action since the first examples were marketed in the 1960s.[2][3] By 2016, at least 18 examples were developed by crop protection companies, with the market leader being boscalid, owing to its broad spectrum of fungal species controlled. However, it lacked full control of important cereal diseases, especially septoria leaf blotch Zymoseptoria tritici.[2]
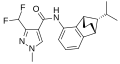
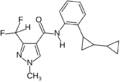
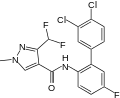
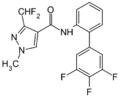
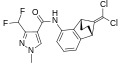
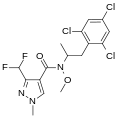
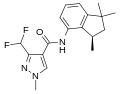
A group of compounds which did control septoria were 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic amides, as shown below,[2][4][5] ordered by year of their first registration.[6]
- Structures of commercial SDHI inhibitors containing the pyrazole
-
Isopyrazam (2010)
-
Sedaxane (2011)
-
Bixafen (2011)
-
Fluxapyroxad (2011)
-
Benzovindiflupyr (2012)
-
Pydiflumetofen (2016)
-
Inpyrfluxam (2019)
Synthesis
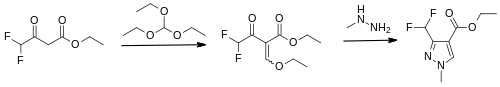
The first reported synthesis of the pyrazole acid was in 1993, by chemists at Monsanto.[7]
The ethyl ester of difluoroacetoacetic acid is treated with triethyl orthoformate in the presence of acetic anhydride[8] and then with methyl hydrazine, which forms mainly the required pyrazole ring, in addition to its isomer with the methyl group on the alternative nitrogen atom. This ester is then hydrolysed with sodium hydroxide to give the pyrazole acid.[7][2]:410
Manufacture of the acid at large scale has been optimised by chemists at Syngenta, Bayer Crop Science and BASF.[2]:409–11
Uses
(As of 2023), amides of the acid were commercialised in seven SDHI fungicides.[2]:406–409,422[6]:Table 3[9]:371–3 The US Geological Survey for 2018 reported that the most heavily used there were fluxapyroxad, at 400,000 pounds (180,000 kg),[10] followed by benzovindiflupyr at 200,000 pounds (91,000 kg).[11] The acid has been reported as a metabolite of fluxapyroxad and pydiflumetofen and thus may be present in the environment where these materials are used.[12][13] The most recently registered example of this class is Sumitomo's inpyrfluxam.[14] Two further compounds, pyrapropoyne (Nissan Chemical Corporation) and flubeneteram (Dongguan Hec Tech) are under development.[6]
This group of pyrazole carboxamide fungicides are very effective against major crop pests such as Alternaria species, including early blight of tomato and potato.[2]:416–8[12] However, none display commercial levels of activity against oomycetes, fungal-related organisms which include important diseases like Phytophthora infestans, late blight of potato.[2]:418[15][16]
References
- ↑ "3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid". 2023-07-22. https://pubchem.ncbi.nlm.nih.gov/compound/18983008.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 Walter, Harald (2016). "Fungicidal Succinate-Dehydrogenase-Inhibiting Carboxamides". Bioactive Carboxylic Compound Classes: Pharmaceuticals and Agrochemicals. Wiley. pp. 405–425. doi:10.1002/9783527693931.ch31. ISBN 9783527339471.
- ↑ "History of SDHI-fungicides". https://www.frac.info/frac-teams/working-groups/sdhi-fungicides/information.
- ↑ "Pyrazolecarboxamide fungicides". BCPC. http://www.bcpcpesticidecompendium.org/class_fungicides.html#pyrazolecarboxamide_fungicides.
- ↑ Walter, Harald; Lamberth, Clemens; Corsi, Camilla (2018). "Synthesis of fungicidally active succinate dehydrogenase inhibitors with novel difluoromethylated heterocyclic acid moieties". Monatshefte für Chemie - Chemical Monthly 149 (4): 791–799. doi:10.1007/s00706-017-2101-y.
- ↑ 6.0 6.1 6.2 Umetsu, Noriharu; Shirai, Yuichi (2020). "Development of novel pesticides in the 21st century". Journal of Pesticide Science 45 (2): 54–74. doi:10.1584/jpestics.D20-201. PMID 33132734.
- ↑ 7.0 7.1 & Metz, S"Pyrazole carboxanilide fungicides and use" US patent 5223526, published 1993-06-29, assigned to Monsanto Co
- ↑ Jones, Reuben G. (1951). "The Synthesis of Ethyl Ethoxymethyleneoxalacetate and Related Compounds". Journal of the American Chemical Society 73 (8): 3684–3686. doi:10.1021/ja01152a034.
- ↑ Jeschke, Peter (2021). "Current Trends in the Design of Fluorine-Containing Agrochemicals". Organofluorine Chemistry. Wiley. pp. 363–395. doi:10.1002/9783527825158.ch11. ISBN 9783527347117.
- ↑ US Geological Survey (2021-10-12). "Estimated Agricultural Use for fluxapyroxad, 2018". https://water.usgs.gov/nawqa/pnsp/usage/maps/show_map.php?year=2018&map=FLUXAPYROXAD&hilo=L&disp=Fluxapyroxad.
- ↑ US Geological Survey (2021-10-12). "Estimated Agricultural Use for benzovindiflupyr, 2018". https://water.usgs.gov/nawqa/pnsp/usage/maps/show_map.php?year=2018&map=BENZOVINDIFLUPYR&hilo=L&disp=Benzovindiflupyr.
- ↑ 12.0 12.1 Pesticide Properties Database. "Fluxapyroxad". University of Hertfordshire. http://sitem.herts.ac.uk/aeru/ppdb/en/Reports/2002.htm.
- ↑ Arena, Maria; Auteri, Domenica; Brancato, Alba et al. (2019). "Peer review of the pesticide risk assessment of the active substance pydiflumetofen". EFSA Journal 17 (10): e05821. doi:10.2903/j.efsa.2019.5821. PMID 32626121.
- ↑ Kiguchi, So; Inoue, Takuya; Matsuzaki, Yuichi; Iwahashi, Fukumatsu; Sakaguchi, Hiroshi (2021). "Discovery and biological profile of inpyrfluxam: A new broad-spectrum succinate dehydrogenase inhibitor fungicide". Recent Highlights in the Discovery and Optimization of Crop Protection Products. pp. 381–389. doi:10.1016/B978-0-12-821035-2.00026-7. ISBN 9780128210352.
- ↑ Pan, Yuemin; Ye, Tao; Gao, Zhimou (2017). "Cloning and functional analysis of succinate dehydrogenase gene PsSDHA in Phytophthora sojae". Microbial Pathogenesis 108: 40–48. doi:10.1016/j.micpath.2017.03.012. PMID 28438637.
- ↑ Wang, Minlong; Du, Ying; Ling, Chen et al. (2021). "Design, synthesis and antifungal/anti-oomycete activity of pyrazolyl oxime ethers as novel potential succinate dehydrogenase inhibitors". Pest Management Science 77 (9): 3910–3920. doi:10.1002/ps.6418. PMID 33871901.
 |