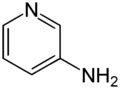
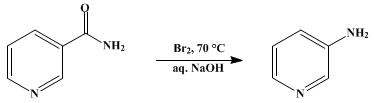Chemistry:3-Aminopyridine
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
Pyridin-3-amine | |
| Other names
3-Pyridinamine; 3-Pyridylamine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| Properties | |
| C5H6N2 | |
| Molar mass | 94.117 g·mol−1 |
| Melting point | 65 °C (149 °F; 338 K) |
| Boiling point | 248 °C (478 °F; 521 K) |
| Soluble[vague] | |
| Solubility in alcohol and benzene | Soluble[vague] |
| Hazards | |
| Flash point | 124 °C (255 °F; 397 K) |
| 628 °C (1,162 °F; 901 K) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
3-Aminopyridine is an aminopyridine. It is a colorless solid.[1]
Preparation
3-Aminopyridine is prepared by heating nicotinamide with sodium hypobromite which is in turn prepared in situ by the reaction of sodium hydroxide and bromine at 70 °C.[2]
It can be used in the synthesis of organic ligand 3-pyridylnicotinamide. Troxipide is another synthesis that uses 3-AP.
Toxicity
The acute toxicity is indicated by the LD50 = 178 mg/kg (quail, oral).[1]
References
- ↑ 1.0 1.1 Shinkichi Shimizu; Nanao Watanabe; Toshiaki Kataoka; Takayuki Shoji; Nobuyuki Abe; Sinji Morishita; Hisao Ichimura (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_399.
- ↑ Allen, C. F. H.; Wolf, Calvin N. (1950). "3-Aminopyridine". Organic Syntheses 30: 3. doi:10.15227/orgsyn.030.0003. http://www.orgsyn.org/demo.aspx?prep=CV4P0045.; Collective Volume, 4, pp. 45
 |