Chemistry:3-Ethyl-3-pentanol
From HandWiki
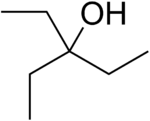
| |
| Names | |
|---|---|
| Preferred IUPAC name
3-Ethylpentan-3-ol | |
| Other names
Triethylcarbinol; 1,1-Diethyl-1-propanol; 3-Ethyl-3-hydroxypentane; Triethylmethanol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C7H16O | |
| Molar mass | 116.204 g·mol−1 |
| Appearance | Clear liquid |
| Density | 0.82 g/cm3 |
| Boiling point | 140–142 °C (284–288 °F; 413–415 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
3-Ethyl-3-pentanol, also known as 3-ethylpentan-3-ol, is a tertiary alcohol with the molecular formula C7H16O.
It reacts with chromic acid by first dehydrating to an olefin 3-ethyl-2-pentene, and then by converting the double bond to an epoxide.[2]
References
- ↑ 3-ethyl-3-pentanol at chemsynthesis.com
- ↑ Sager, W. F. (October 1956). "The Chromic Acid Oxidation of 3-Ethyl-3-pentanol". Journal of the American Chemical Society 78 (19): 4970–4972. doi:10.1021/ja01600a045.
 |

