Chemistry:4-ANPP
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
N-Phenyl-1-(2-phenylethyl)piperidin-4-amine | |
| Other names
desproprionyl fentanyl; 4-anilino-N-phenethylpiperidine; 4-ANPP; ANPP
| |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | ANPP |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C19H24N2 | |
| Molar mass | 280.415 g·mol−1 |
| Legal status | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
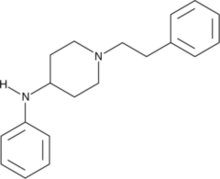
4-ANPP, also known as 4-anilino-N-phenethylpiperidine (4-ANPP), 4-aminophenyl-1-phenethylpiperidine, or despropionyl fentanyl,[2] is a direct precursor to fentanyl and some fentanyl analogues such as acetylfentanyl. It is commonly found as a contaminant in samples of drugs containing fentanyl, which may include samples represented by the supplier as heroin or other opioids.[3] It is not psychoactive and is present only as a result of improper processing of the intended product of the synthesis.
Preparation
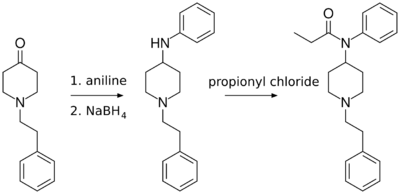
4-ANPP can be prepared from N-phenethyl-4-piperidinone (NPP) and aniline, then being reduced.
4-ANPP can also be prepared using 4-anilinopiperidine and selectively adding the phenethyl group.
Uses
4-ANPP is useful in the synthesis of pharmaceuticals, primarily fentanyl and its analogs. Paul Janssen (founder of Janssen Pharmaceutica) first synthesized fentanyl in 1960 using a similar method, with Benzylfentanyl as an intermediate.[4] The following synthesis, developed by an individual under the pseudonym of Siegfried, involves the reductive amination of N-phenethyl-4-piperidinone (NPP) to 4-ANPP. This product is reacted with propionyl chloride to form fentanyl.
See also
References
- ↑ "Red list". https://www.incb.org/incb/en/precursors/Red_Forms/red-list.html.
- ↑ "4-ANPP" (in en). https://www.caymanchem.com/product/18810.
- ↑ DrugsData.org. "DrugsData.org: Results : Lab Test Results for Fentanyl". https://www.drugsdata.org/search.php?substance1=2021&substance2=2344.
- ↑ Schulz, William. "Fentanyl". List of Top Pharmaceuticals. Chemical & Engineering News. http://pubs.acs.org/cen/coverstory/83/8325/8325fentanyl.html.
 |