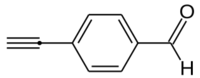
Chemistry:4-Ethynylbenzaldehyde
From HandWiki
| |
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
4-Ethynylbenzaldehyde | |
| Other names
p-Ethynylbenzaldehyde
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C9H6O | |
| Molar mass | 130.146 g·mol−1 |
| Appearance | white or yellow solid |
| Melting point | 89–93 °C (192–199 °F; 362–366 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
4-Ethynylbenzaldehyde, or p-ethynylbenzaldehyde, is an organic compound with the formula HC2C6H4COH.[2] It is an ethynyl derivative of benzaldehyde, or may also be viewed as a formylated derivative of phenylacetylene.
Preparation
4-Ethynylbenzaldehyde may be prepared by the Sonogashira coupling of 4-bromobenzaldehyde with trimethylsilylacetylene to form 4-((trimethylsilyl)ethynyl)benzaldehyde, followed by removal of the trimethylsilyl group with base to form 4-ethynylbenzaldehyde.[3]
Reactions
The ethynyl functionality of 4-ethynylbenzaldehyde may undergo a Sonogashira coupling with another molecule of 4-bromobenzaldehyde to form the symmetrical dialdehyde 4,4'-(ethyne-1,2-diyl)dibenzaldehyde.[3]
References
- ↑ "4-Ethynylbenzaldehyde". https://www.chemspider.com/Chemical-Structure.2052086.html.
- ↑ PubChem. "4-Ethynylbenzaldehyde" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/4-Ethynylbenzaldehyde.
- ↑ 3.0 3.1 Xu, X.; Cai, P.; Chen, H.; Zhou, H.-C.; Huang, N. (28 September 2022). "Three-Dimensional Covalent Organic Frameworks with she Topology". Journal of the American Chemical Society 144 (40): 18511–18517. doi:10.1021/jacs.2c07733. PMID 36170014.
 |


