Chemistry:4-Fluoroselegiline
From HandWiki
Short description: Chemical compound
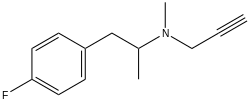 | |
| Clinical data | |
|---|---|
| Trade names | Fludepryl |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C13H16FN |
| Molar mass | 205.276 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.024 ± 0.06 g/cm3 |
| Boiling point | 276.2 ± 25 °C (529.2 ± 45.0 °F) |
| |
| |
4-Fluoroselegiline or p-fluoro-L-deprenyl is a substituted amphetamine designer drug. Much like its parent compount, selegiline, it is a selective and irreversible inhibitor of monoamine oxidase B.[1]
Pharmacology
Pharmacodynamics
4-Fluoroselegiline has a similar pharmacological profile to its parent compound, selegiline.[2]
A radiolabelled derivative incorporating 18F is used to study MAO-B inhibition in both in vivo and in vitro experiments.[3]
Pharmacokinetics
p-Fluoro-L-deprenyl is metabolized to p-fluoro-L-methamphetamine and p-fluoro-L-amphetamine, both of which are active. The levels of substituted amphetamine metabolites in the brain is three times higher following 4-fluoroselegiline administration compared to an equivalent dose of selegiline.[2]
References
- ↑ Erdaö, Franciska; Baranyi, Attila; Takács, József; Arányi, Péter (3 August 2000). "Different neurorescue profiles of selegiline and p-fluoro-selegiline in gerbils". NeuroReport 11 (11): 2597–3100. doi:10.1097/00001756-200008030-00049. ISSN 0959-4965. PMID 10943729. https://journals.lww.com/neuroreport/abstract/2000/08030/different_neurorescue_profiles_of_selegiline_and.49.aspx. Retrieved 4 January 2024.
- ↑ 2.0 2.1 Yasar, Sevil; Gaal, Jozsef; Justinova, Zuzana; Bergman, Jack (1 October 2005). "Discriminative stimulus and reinforcing effects of p-fluoro-l-deprenyl in monkeys" (in en). Psychopharmacology 182 (1): 95–103. doi:10.1007/s00213-005-0063-y. ISSN 1432-2072. PMID 15990999. https://link.springer.com/article/10.1007/s00213-005-0063-y.
- ↑ Plenevaux, Alain; Fowler, Joanna S.; Dewey, Stephen L.; Wolf, Alfred P.; Guillaume, Marcel (January 1991). "The synthesis of no-c arrier-added DL-4-[18F]fluorodeprenyl via the nucleophilic aromatic substitution reaction". International Journal of Radiation Applications and Instrumentation. Part A. Applied Radiation and Isotopes 42 (2): 121–127. doi:10.1016/0883-2889(91)90060-E. PMID 1648033.
 |

