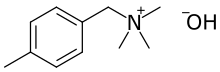
Chemistry:4-Methylbenzyltrimethylammonium hydroxide
From HandWiki
| |
| Names | |
|---|---|
| Other names
p-Methylbenzyltrimethylammonium hydroxide
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| C11H19NO | |
| Molar mass | 181.279 g·mol−1 |
| Boiling point | dec. |
| Related compounds | |
Related compounds
|
Benzyltrimethylammonium hydroxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
4-Methylbenzyltrimethylammonium hydroxide is a quaternary ammonium compound with the formula C11H18N+OH−. It can be synthesized by reacting 4-methylbenzyl bromide with triethylamine, followed by stirring with silver oxide in water.[1]
Reactions
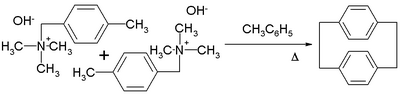
When heated with phenothiazine in toluene, it undergoes Hofmann elimination to form [2.2]Paracyclophane.[1]
Paracycloheterophanes can be obtained by heating it with other quaternary ammonium hydroxides (e.g. with (5-methyl-2-thienylmethyl)trimethylammonium hydroxide to form [2.2]paracyclo(2,5)thiophenophane).[2]
References
- ↑ 1.0 1.1 H. E. Winberg, F. S. Fawcett (1962). "[2.2]Paracyclophane". Organic Syntheses 42: 83. doi:10.15227/orgsyn.042.0083.
- ↑ Otsubo, T., Mizogami, S., Osaka, N., Sakata, Y., & Misumi, S. (1977). Layered Compounds. XLII. Syntheses and Properties of Layered Paracycloheterophanes. Bulletin of the Chemical Society of Japan, 50(7), 1841–1849. doi:10.1246/bcsj.50.1841
 |