Chemistry:6:2-Fluorotelomersulfonic acid
From HandWiki
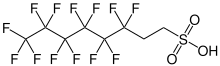
| |
| Names | |
|---|---|
| Other names
6:2 Fluorotelomer Sulfonate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H5F13O3S | |
| Molar mass | 428.16 g·mol−1 |
| Appearance | White to light brown solid |
| Density | 1.953 g/cm³ (at 20 °C) |
| Melting point | 87 °C (189 °F; 360 K) |
| 58 g/l in water (20 °C) | |
| Acidity (pKa) | 1.31 |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H302, H314, H373 | |
| P260, P264, P264+265Script error: No such module "Preview warning".Category:GHS errors, P270, P280, P301+317Script error: No such module "Preview warning".Category:GHS errors, P301+330+331, P302+361+354Script error: No such module "Preview warning".Category:GHS errors, P304+340, P305+354+338Script error: No such module "Preview warning".Category:GHS errors, P316Script error: No such module "Preview warning".Category:GHS errors, P317Script error: No such module "Preview warning".Category:GHS errors, P319Script error: No such module "Preview warning".Category:GHS errors, P321, P330, P363, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
6:2-fluorotelomersulfonic acid (6:2-FTS) is a chemical compound that belongs to the group of fluorotelomersulfonic acids within the broader class of per- and polyfluorinated alkyl compounds (PFAS). Due to its structural similarity to perfluorooctanesulfonic acid (PFOS), it is also called H 4 PFOS .[2]
Production
6:2-Fluorotelomersulfonic acid is produced via telomerization. In addition, it is also formed, for example, from some perfluorocarboxybetaines after their splitting.[3]
Usage
6:2-Fluorotelomersulfonic acid and its derivatives are used as a replacement product for perfluorooctanesulfonic acid (PFOS) or its salts in fire-fighting foams.
References
- ↑ "1-Octanesulfonic acid, 3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluoro-" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/119688#section=Safety-and-Hazards.
- ↑ "Comparison of the chemical structural formula of 6:2 FTCA/6:2 FTSA and... | Download Scientific Diagram". https://www.researchgate.net/figure/Comparison-of-the-chemical-structural-formula-of-62-FTCA-62-FTSA-and-PFOA-PFOS_fig1_311957588.
- ↑ "zwitterionic carboxybetaine polymer: Topics by Science.gov". https://www.science.gov/topicpages/z/zwitterionic+carboxybetaine+polymer.
 |

