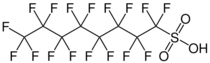
Chemistry:Perfluorooctanesulfonic acid
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-Heptadecafluorooctane-1-sulfonic acid | |
| Other names
PFOS
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8HF17O3S | |
| Molar mass | 500.13 g/mol |
| Boiling point | 133 °C (271 °F; 406 K) at 6 torr |
| Acidity (pKa) | <<0[1][2] |
| Hazards | |
| Main hazards | Toxic, persistent environmental pollutant |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| NFPA 704 (fire diamond) | |
| Pharmacology | |
| Legal status |
|
| Related compounds | |
Related compounds
|
Perfluorooctanoic acid (PFOA), Perfluorobutanesulfonic acid (PFBS), Perfluorooctanesulfonamide (PFOSA), Perfluorononanoic acid (PFNA) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Perfluorooctanesulfonic acid (PFOS) (conjugate base perfluorooctanesulfonate) is a chemical compound having an eight-carbon fluorocarbon chain and a sulfonic acid functional group and thus a perfluorosulfonic acid. It is an anthropogenic (man-made) fluorosurfactant, now regarded as a global pollutant. PFOS was the key ingredient in Scotchgard, a fabric protector made by 3M, and related stain repellents. The acronym "PFOS" refers to the parent sulfonic acid and to various salts of perfluorooctanesulfonate. These are all colorless or white, water-soluble solids. Although of low acute toxicity, PFOS has attracted much attention for its pervasiveness and environmental impact. It was added to Annex B of the Stockholm Convention on Persistent Organic Pollutants in May 2009.[4]
History
In 1949, 3M began producing PFOS-based compounds by electrochemical fluorination resulting in the synthetic precursor perfluorooctanesulfonyl fluoride.[5] In 1968, organofluorine content was detected in the blood serum of consumers, and in 1976 it was suggested to be perfluorooctanoic acid (PFOA) or a related compound such as PFOS.[6][7][8] In 1997, 3M detected PFOS in blood from global blood banks,[9] although the company's internal documents indicate knowledge of this decades earlier, dating from the 1970s.[10] In 1999, the U.S. Environmental Protection Agency began investigating perfluorinated compounds after receiving data on the global distribution and toxicity of PFOS, the key ingredient in Scotchgard.[11] For these reasons, and USEPA pressure,[12] the primary American producer of PFOS, 3M, announced, in May 2000, the phaseout of the production of PFOS, PFOA, and PFOS-related products.[13][10] Most other manufacturers (particularly, those in Europe) phased out the production of PFOS and perfluorooctanoic acid (PFOA) in 2000 and 2006, respectively. A shorter-chain PFOS (perfluorohexanesulfonic acid, PFHxS), was included in Annex A to the Stockholm Convention in 2022.[14]
Currently, most of PFOS and PFOS-related chemicals are produced in China.[15]
Chemistry
The main method used for the industrial scale production of PFOS is electrochemical fluorination (ECF).[16] ECF is an electrolysis production method where a precursor of perfluorooctanesulfonyl fluoride is dispersed in a solution of hydrogen fluoride and electrolyzed. This production method, whilst economic and mainly results in PFOS, also results in shorter chain perfluoroalkyl substances being formed. PFOS predominates in the resultant mixture, however, if the reaction is allowed to continue this begins to favor the production of shorter chain PFAS. A distinct isomer ratio has been observed in PFOS produced by ECF, in the order of 70% linear PFOS, 25% branched and 5% terminal; this is not a function of the production process but rather that the precursor also exhibits this isomer ratio. ECF was the means by which 3M produced PFOS up until May 2000 when the company announced a phaseout of fluorosurfactants.
89 constitutional isomers of PFOS are theoretically possible, environmental samples usually contain a mixture of the linear isomer and 10 branched isomers.[17]
Telomerisation involves constructing the PFOS molecule using short chain (often 2-carbon) precursors and adding a sulfonate group as a final step. This production process results in 100% linear PFOS. This production method, whilst cleaner and resulting in a much more pure product than ECF, is not known to have been widely used except for the production of reagent grade PFOS and analytical standards.
Indirect routes
Perfluorooctylsulfonyl compounds degrade to PFOS.[18] Examples include N-methyl perfluorooctane sulfonamidoethanol (N-MeFOSE), a carpet stain repellent, and N-ethyl perfluorooctane sulfonamidoethanol (N-EtFOSE), a paper treatment.[19] Also perfluorooctanesulfonamide is a precursor.[20] About 50 precursors were named in the 2004 proposed Canada ban on PFOS.[21]
Degradation
PFOS virtually does not degrade under environmental conditions and is thus highly persistent. Waste water treatment plants are also unable to degrade PFOS.[22] On the other hand, precursors are transformed to PFOS in waste water treatment plants.[23]
Properties
The C8F17 subunit of PFOS is hydrophobic and lipophobic, like other fluorocarbons, while the sulfonic acid/sulfonate group adds polarity. PFOS is an exceptionally stable compound in industrial applications and in the environment because of the effect of aggregate carbon–fluorine bonds. PFOS is a fluorosurfactant that lowers the surface tension of water more than that of hydrocarbon surfactants.
Uses
Perfluorooctanesulfonic acid is usually used as the sodium or potassium salts.
- PFOS was the key ingredient in Scotchgard, a fabric protector made by 3M, and numerous stain repellents.
- PFOS, together with PFOA, has also been used to make aqueous film forming foam (AFFF), a component of fire-fighting foams, and alcohol-type concentrate foams.
- PFOS compounds can also be found in some impregnation agents for textiles, paper, and leather; in wax, polishes, paints, varnishes, and cleaning products for general use; in metal surfaces, and carpets.
- In the semiconductor industry, PFOS is used in multiple photolithographic chemicals including: photoacid generators (PAGs) and anti-reflective coatings (ARCs). It has been phased out in the European Union semiconductor industry due to health concerns.
- PFOS is the key ingredient in Skydrol, a fire-resistant hydraulic fluid used in commercial aviation.
The most important emission sources of PFOS are metal plating and fire-fighting foams.[24] Because of concerns about PFOS, F-53B has been used as a replacement for mist suppression in metal plating.[25]
Levels in humans
Because of its chemical nature, PFOS will remain in the body for several years. It is estimated that it takes 4 years for half of this substance to be eliminated from the body.[26]
PFOS is detected in the blood serum of almost all people in the U.S., but concentrations have been decreasing over time. In contrast, PFOS blood levels appear to be rising in China[27] where PFOS production continues. A study of ca. 2000 teenagers from 9 European countries with most samples collected in years 2016-2018 found higher blood concentrations of several PFOS’s in those, who consumed more seafood, eggs or offal, as well as in those from North and West (versus the South and East) Europe. Within the same country, boys had a higher PFOS concentrations than girls. A typical PFOS blood concentration range in this study was 1,500-2,500 ppb. [28]
Much higher levels of blood PFOS (12,830 ppb) have been reported in people with occupational exposure [29]—or possibly 1,656 parts per billion[30]—in a consumer. Occupationally exposed individuals may have an average level of PFOS over 1000 parts per billion, and a small segment of individuals in the upper range of the general population may be over the 91.5 parts per billion level.[31]
PFOS exposure has been demonstrated as early as fetal development during pregnancy since PFOS can easily pass through the placenta.[32] It has been shown that fetal exposure to PFOS is quite prevalent and has been shown to be detected in greater than 99% of umbilical cord serum samples.[33]
PFOS has been detected in U.S. freshwater fish,[34][35] as well as in municipal wastewater[36] and drinking water samples,[37] worldwide, at concentrations ranging between few ng/L and some μg/L.
Levels in wildlife
A variety of wildlife species have had PFOS levels measured in egg, liver, kidney, serum, and plasma samples and some of the highest recorded values as of January 2006 are listed below.[38]
| Species | Geography | Year | Sample | PFOS (ppb) |
|---|---|---|---|---|
| Bald eagle | Midwestern United States | 1990–93 | plasma | 2,200 |
| Brandt's cormorant | California , US | 1997 | liver | 970 |
| Guillemot | Baltic Sea, Sweden | 1997 | egg | 614 |
| Carrion crow | Tokyo Bay, Japan | 2000 | liver | 464 |
| Red-throated loon | North Carolina, US | 1998 | liver | 861 |
| Polar bear | Sanikiluaq, Nunavut, Canada | 2002 | liver | 3,100 |
| Harbor seal | Wadden Sea, the Netherlands | 2002 | muscle | 2,725 |
| Bottlenose dolphin | Charleston, South Carolina, US | 2003 | plasma | 1,315 |
| Common dolphin | Mediterranean Sea, Italy | 1998 | liver | 940 |
| Mink | Michigan, US | 2000–01 | liver | 59,500 |
| Common shiner | Ontario, Canada | 2001 | liver | 72,900 |
| Great tit | near 3M, Port of Antwerp, Belgium | 2007 | liver | 553–11,359[39] |
Despite the global wide-ranging restriction, PFOS concentrations in air continued to increase at many monitoring stations between 2009 and 2017.[40]
Health effects in humans and wildlife
There has been a growing body of evidence investigating the health effects of PFOS on the reproductive, developmental, liver, kidney, thyroid, and immunological effects in humans.[41]
Pregnancy outcomes
Several studies have focused on pregnancy outcomes in infants and mothers who are exposed to PFOS during pregnancy. For developing offspring, exposure to PFOS occurs through the placenta.[32] While the impact of PFOS compounds on fetal development continues to be an ongoing investigation, findings have demonstrated a relationship between PFOS exposure in pregnant mothers and negative birth outcomes.[42]
There has been some evidence to suggest that PFOS levels in pregnant women have been associated with preeclampsia, preterm labor, low birth weight and gestational diabetes.[43][44] Although, the strongest association is between PFOS levels with preterm birth and preeclampsia.[44][45] There has been some evidence to suggest that PFOS impairs fetal growth during pregnancy, although findings have been inconsistent.[44]
The specific physiological mechanisms behind adverse pregnancy outcomes with PFOS exposure remain unclear. One proposed cause has to do with PFOS impairment on placental blood flow.[41] This mechanism could help explain several of the pregnancy-related outcomes from PFOS exposure including such as intrauterine growth development, low birth weight, preterm birth labor, and preeclampsia. Additional physiological mechanisms may include disruption in inflammatory signals during pregnancy, decreased trophoblast signaling and trophoblast migration.[46] Additionally, PFOS exposure has been shown to be related to the downregulation genes corresponding to growth factors, pregnancy-related signal transducers, and maternal hormones.[47] PFOS impact on thyroid hormone regulation also has the potential to impact several birth outcomes.[48][49]
Breastfeeding and lactation
PFOS has been measured in breastmilk and is estimated to contribute the greatest level of PFOS exposure in infants. Specifically, the duration of breastfeeding has been shown to be associated with increases in PFOS in infants.[50] Some evidence has shown that breastmilk provides more than 94% of the PFOS exposure in infants up to six months old.[51] The Agency for Toxic Substances and Disease Registry (ATSDR) concluded that breastfeeding benefits continue to outweigh potential risks associated with PFOS in breastmilk.[52]
Infertility
PFAS compounds such as PFOS act as an endocrine disruptor of the reproductive system.[53] As such, there is concern over the impact of this compound on fertility. There is some evidence to suggest that PFOS may impair fertility in both females and males. One study found that women with higher levels of PFOS and PFOA took longer to become pregnant than those with lower levels, suggesting that the chemicals may impair fertility.[54] The impact of PFOS on male fertility is still under investigation. There have been some studies that demonstrated that PFOS is associated with a decrease in sperm count and as well as a decrease in the number of morphologically normal sperm.[33] There has also been evidence to suggest that PFOS may also reduce testosterone levels.[33]
Thyroid disease
Increased levels of PFOS have been shown to accumulate in thyroid gland cells and have been associated with altered thyroid hormone levels in adults.[55][56] Appropriate levels of thyroid hormone during pregnancy are critical for a developing fetus as this hormone is involved with brain development and body growth.[57] Studies have demonstrated a relationship between PFOS exposure and thyroid dysfunction during pregnancy resulting in altered thyroid hormone levels in both the mother and the fetus.[58][59]
Hypercholesterolemia
PFOS has been associated with increased risk of abnormal levels of cholesterol.[60][61][62] Specifically, epidemiological studies in humans have reported an association between increased PFOS levels and the total cholesterol and low density lipoprotein (LDL) cholesterol.[63]
ADHD
Levels of PFOS in US children aged 12–15 were associated with an increased risk (60% over the interquartile range) of attention deficit hyperactivity disorder (ADHD).[64] The importance of exposure timing during development is unclear, however, some evidence has shown that exposure to PFOS during fetal development was not associated with an increased risk for developing of ADHD later in childhood.[65]
Chronic kidney disease
Serum levels of PFOS were found to be associated with increased risk of chronic kidney disease in the general US population.[66] "This association was independent of confounders such as age, sex, race/ethnicity, body mass index, diabetes, hypertension, and serum cholesterol level."[66] According to a 2002 study by the Environmental Directorate of the OECD, "PFOS is persistent, bioaccumulative, and toxic to mammalian species."[67]
Cancer
Research demonstrating the association between PFOS and cancer is still ongoing. A few studies have demonstrated an elevated risk for prostate and bladder cancer, however, there were notable limitations in the design and analysis of these studies.[44] As of November 2023, the International Agency for Research on Cancer (IARC) has classified PFOS as possibly carcinogenic to humans (Group 2b) based on “strong” mechanistic evidence.[68] The Division of Cancer Epidemiology & Genetics (DCEG) is currently investigating the association of several PFAS compounds and cancers including kidney cancer, testicular cancer, prostate cancer, ovarian and endometrial cancer, thyroid cancer, non-hodgkins lymphoma, and childhood leukemia.[69]
In wildlife
The levels observed in wild animals are considered sufficient to "alter health parameters".[70][71]
PFOS affects the immune system of male mice at a blood serum concentration of 91.5 parts per billion, raising the possibility that highly exposed people and wildlife are immunocompromised.[31] Chicken eggs dosed at 1 milligram per kilogram (or 1 part per million) of egg weight developed into juvenile chickens with an average of ~150 parts per billion in blood serum—and showed brain asymmetry and decreased immunoglobulin levels.[72]
Regulation
Globally
It was added to Annex B of the Stockholm Convention on Persistent Organic Pollutants in May 2009.[4] Originally, parties agreed on acceptable proposes (time-unlimited exemptions) for the following uses—in addition to a range of specific exemptions (time-limited):[73]
- Photo-imaging
- Photo-resist and anti-reflective coatings for semi-conductors
- Etching agent for compound semi-conductors and ceramic filters
- Aviation hydraulic fluids
- Metal plating (hard metal plating) only in closed-loop systems
- Certain medical devices (such as ethylene tetrafluoroethylene copolymer (ETFE) layers and radio-opaque ETFE production, in-vitro diagnostic medical devices, and CCD colour filters)
- Fire-fighting foam
- Insect baits for control of leaf-cutting ants from Atta spp. and Acromyrmex spp.
In 2019, it was decided to only keep one acceptable purpose:[74]
- Insect baits with sulfluramid (CAS No. 4151-50-2) as an active ingredient for control of leaf-cutting ants from Atta spp. and Acromyrmex spp. for agricultural use only
Canada
In 2023, the Government of Canada is considering addressing PFAS as a class rather than as individual substances or in smaller groups. A report to conclude that PFAS as a class are harmful to human health and the environment, and to define risk management aspects and alternatives to PFAs, is under development."Per-and polyfluoroalkyl substances (PFAS)"
Europe
Based on an OECD study on PFOS[67] and a risk assessment by Europe's Scientific Committee on Health and Environmental Risks[75] the European Union practically banned the use of PFOS in finished and semi-finished products in 2006 (maximum content of PFOS: 0.005% by weight).[76] However, PFOS use for industrial applications (e.g. photolithography, mist suppressants for hard chromium plating, hydraulic fluids for aviation) was exempted. In 2009 this directive was incorporated into the REACH regulation.[77] In the summer of 2010 PFOS was added to the regulation on persistent organic pollutants and the threshold was lowered to max. 0.001% by weight (10 mg/kg).[78]
United States
In 2018 the State of Michigan established a legally enforceable groundwater cleanup level of 70 ppt for both PFOA and PFOS.[79]
In 2020 the Michigan Department of Environment, Great Lakes, and Energy (EGLE) adopted stricter drinking water standards in the form of maximum contaminant levels (MCLs), lowering acceptable levels from the 2018 enforceable groundwater cleanup levels of 70 ppt to 8 ppt for PFOA and 16 ppt for PFOS and adding MCLs for 5 previously unregulated PFAS compounds PFNA, PFHxA, PFHxS, PFBS, and HFPO-DA.[80][81]
In 2020, a California bill was passed banning PFOS and the following salts as an intentionally added ingredient from cosmetics: ammonium perfluorooctane sulfonate, diethanolamine perfluorooctane sulfonate, lithium perfluorooctane sulfonate and potassium perfluorooctane sulfonate.[82]
In March 2021 the U.S. EPA announced that it will develop national drinking water standards for PFOA and PFOS.[83]
In October 2021 the EPA proposed to designate PFOA and PFOS as hazardous substances in its PFAS Strategic Roadmap.[84][85] In September 2022 the EPA proposed to designate as hazardous substances under the Superfund Comprehensive Environmental Response, Compensation, and Liability Act of 1980 (CERCLA).
See also
- 2005 Hertfordshire Oil Storage Terminal fire
- Fluorocarbons
- Per- and polyfluoroalkyl substances
- Timeline of events related to per- and polyfluoroalkyl substances
References
- ↑ "Acid dissociation versus molecular association of perfluoroalkyl oxoacids: Environmental implications". J. Phys. Chem. A 113 (29): 8152–8156. July 2009. doi:10.1021/jp9051352. PMID 19569653. Bibcode: 2009JPCA..113.8152C. https://authors.library.caltech.edu/15048/2/jp9051352_si_001.pdf.
- ↑ "Extending the semi-empirical PM6 method for carbon oxyacid pKa prediction to sulfonic acids: Application towards congener-specific estimates for the environmentally and toxicologically relevant C1 through C8 perfluoroalkyl derivatives". Nature Precedings: 1. 2009. doi:10.1038/npre.2009.3011.
- ↑ An Act To Stop Perfluoroalkyl and Polyfluoroalkyl Substances Pollution. 130th Maine Legislature, April 15, 2021
- ↑ 4.0 4.1 Governments unite to step-up reduction on global DDT reliance and add nine new chemicals under international treaty. Geneva: Stockholm Convention Secretariat. 8 May 2009. http://chm.pops.int/Convention/Pressrelease/COP4Geneva8May2009/tabid/542/language/en-US/Default.aspx.
- ↑ "A first global production, emission, and environmental inventory for perfluorooctane sulfonate". Environ. Sci. Technol. 43 (2): 386–92. January 2009. doi:10.1021/es802216n. PMID 19238969. Bibcode: 2009EnST...43..386P.
- ↑ "The toxicology of perfluorooctanoate". Crit. Rev. Toxicol. 34 (4): 351–84. 2004. doi:10.1080/10408440490464705. PMID 15328768.
- ↑ "Perfluorochemical surfactants in the environment". Environ. Sci. Technol. 36 (7): 146A–152A. April 2002. doi:10.1021/es022253t. PMID 11999053. Bibcode: 2002EnST...36..146G.
- ↑ "The developmental toxicity of perfluoroalkyl acids and their derivatives". Toxicol. Appl. Pharmacol. 198 (2): 231–41. July 2004. doi:10.1016/j.taap.2003.11.031. PMID 15236955. https://zenodo.org/record/1259371.
- ↑ "The Inside Story: 3M and Scotchgard". Environmental Working Group. http://www.chemicalindustryarchives.org/dirtysecrets/scotchgard/1.asp.
- ↑ 10.0 10.1 Fellner, Carrie (16 June 2018). "Toxic Secrets: Professor 'bragged about burying bad science' on 3M chemicals". Sydney Morning Herald. https://www.smh.com.au/lifestyle/health-and-wellness/toxic-secrets-professor-bragged-about-burying-bad-science-on-3m-chemicals-20180615-p4zlsc.html.
- ↑ Ullah, Aziz (October 2006). "The Fluorochemical Dilemma: What the PFOS/PFOA fuss is all about". Cleaning & Restoration. https://www.restorationindustry.org/buyersguide/FlurochemicalsOct06.pdf.
- ↑ "E.P.A. Orders Companies to Examine Effects of Chemicals". The New York Times. 15 April 2003. https://www.nytimes.com/2003/04/15/science/epa-orders-companies-to-examine-effects-of-chemicals.html?pagewanted=2.
- ↑ 3M: "PFOS-PFOA Information: What is 3M Doing?" Accessed October 25, 2008.
- ↑ Richterová, D.; Govarts, E.; Fábelová, L.; Rausová, K.; Rodriguez Martin, L.; Gilles, L.; Remy, S.; Colles, A. et al. (2023). "PFAS levels and determinants of variability in exposure in European teenagers – Results from the HBM4EU aligned studies (2014–2021)" (in en). International Journal of Hygiene and Environmental Health 247: 114057. doi:10.1016/j.ijheh.2022.114057. PMID 36327670.
- ↑ Wang, Tieyu; Wang, Pei; Meng, Jing; Liu, Shijie; Lu, Yonglong; Khim, Jong Seong; Giesy, John P. (2015). "A review of sources, multimedia distribution and health risks of perfluoroalkyl acids (PFAAs) in China". Chemosphere 129: 87–99. doi:10.1016/j.chemosphere.2014.09.021. PMID 25262946. Bibcode: 2015Chmsp.129...87W. http://ir.rcees.ac.cn/handle/311016/32241.
- ↑ Risk profile on perfluorooctane sulfonate. POPs Review Committee. 2006. http://www.pops.int/Portals/0/download.aspx?d=UNEP-POPS-POPRC.2-17-Add.5.English.pdf.
- ↑ Buck, Robert C; Franklin, James; Berger, Urs; Conder, Jason M; Cousins, Ian T; de Voogt, Pim; Jensen, Allan Astrup; Kannan, Kurunthachalam et al. (2011). "Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins". Integrated Environmental Assessment and Management 7 (4): 513–541. doi:10.1002/ieam.258. PMID 21793199.
- ↑ Lists of PFOS, PFAS, PFCA, related compounds and chemicals that may degrade to PFCA. OECD. August 2007. http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?doclanguage=en&cote=env/jm/mono%282006%2915.
- ↑ Renner R (March 2004). "Perfluorinated sources outside and inside". Environ. Sci. Technol. 38 (5): 80A. doi:10.1021/es040387w. PMID 15046317. Bibcode: 2004EnST...38...80R.
- ↑ Lehmler, HJ (March 2005). "Synthesis of environmentally relevant fluorinated surfactants—a review". Chemosphere 58 (11): 1471–96. doi:10.1016/j.chemosphere.2004.11.078. PMID 15694468. Bibcode: 2005Chmsp..58.1471L.
- ↑ Pelley J (December 2004). "Canada moves to eliminate PFOS stain repellents". Environ. Sci. Technol. 38 (23): 452A. doi:10.1021/es040676k. PMID 15597866.
- ↑ Trojanowicz, Marek; Bojanowska-Czajka, Anna; Bartosiewicz, Iwona; Kulisa, Krzysztof (2018). "Advanced Oxidation/Reduction Processes treatment for aqueous perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS) – A review of recent advances". Chemical Engineering Journal 336: 170–199. doi:10.1016/j.cej.2017.10.153.
- ↑ Eriksson, Ulrika; Haglund, Peter; Kärrman, Anna (2017). "Contribution of precursor compounds to the release of per- and polyfluoroalkyl substances (PFASs) from waste water treatment plants (WWTPs)". Journal of Environmental Sciences (China) 61: 80–90. doi:10.1016/j.jes.2017.05.004. PMID 29191318.
- ↑ "Federal Office for the Environment - Homepage". https://www.bafu.admin.ch/uw-0922-e.
- ↑ Yang, Renjun; Liu, Shuyu; Liang, Xiaoxing; Yin, Nuoya; Ruan, Ting; Jiang, Linshu; Faiola, Francesco (June 2020). "F–53B and PFOS treatments skew human embryonic stem cell in vitro cardiac differentiation towards epicardial cells by partly disrupting the WNT signaling pathway". Environmental Pollution 261: 114153. doi:10.1016/j.envpol.2020.114153. PMID 32088431. https://pubmed.ncbi.nlm.nih.gov/32088431/. Retrieved 20 December 2022.
- ↑ "ATSDR Public Health Statement Perfluoroalkyls". https://www.atsdr.cdc.gov/toxprofiles/tp200-c1-b.pdf.
- ↑ Renner, Rebecca (2008). "PFOS phaseout pays off". Environ. Sci. Technol. 42 (13): 4618. doi:10.1021/es0871614. PMID 18677976. Bibcode: 2008EnST...42.4618R.
- ↑ PFAS levels and determinants of variability in exposure in European teenagers – Results from the HBM4EU aligned studies (2014–2021). 2023. Int J Hyg Environ Health. 247/. D. Richterová, E. Govarts, L. Fábelová, K. Rausová, L. Rodriguez Martin, L. Gilles, et al. doi: 10.1016/j.ijheh.2022.114057.
- ↑ "Perfluorinated compounds—exposure assessment for the general population in Western countries". Int. J. Hyg. Environ. Health 212 (3): 239–70. May 2009. doi:10.1016/j.ijheh.2008.04.007. PMID 18565792.
- ↑ Olsen, Geary W; Church, Timothy R; Miller, John P; Burris, Jean M; Hansen, Kristen J; Lundberg, James K; Armitage, John B; Herron, Ross M et al. (December 2003). "Perfluorooctanesulfonate and other fluorochemicals in the serum of American Red Cross adult blood donors.". Environmental Health Perspectives 111 (16): 1892–1901. doi:10.1289/ehp.6316. PMID 14644663.
- ↑ 31.0 31.1 Betts KS (July 2008). "Not immune to PFOS effects?". Environ. Health Perspect. 116 (7): A290. doi:10.1289/ehp.116-a290a. PMID 18629339.
- ↑ 32.0 32.1 Sunderland, Elsie M.; Hu, Xindi C.; Dassuncao, Clifton; Tokranov, Andrea K.; Wagner, Charlotte C.; Allen, Joseph G. (March 2019). "A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects". Journal of Exposure Science & Environmental Epidemiology 29 (2): 131–147. doi:10.1038/s41370-018-0094-1. ISSN 1559-064X. PMID 30470793.
- ↑ 33.0 33.1 33.2 Tarapore, Pheruza; Ouyang, Bin (2021-04-05). "Perfluoroalkyl Chemicals and Male Reproductive Health: Do PFOA and PFOS Increase Risk for Male Infertility?". International Journal of Environmental Research and Public Health 18 (7): 3794. doi:10.3390/ijerph18073794. ISSN 1661-7827. PMID 33916482.
- ↑ LaMotte, Sandee (17 January 2023). "Locally caught fish are full of dangerous chemicals called PFAS, study finds" (in en). CNN. https://edition.cnn.com/2023/01/17/health/freshwater-fish-pfas-contamination-wellness/index.html.
- ↑ Barbo, Nadia; Stoiber, Tasha; Naidenko, Olga V.; Andrews, David Q. (1 March 2023). "Locally caught freshwater fish across the United States are likely a significant source of exposure to PFOS and other perfluorinated compounds" (in en). Environmental Research 220: 115165. doi:10.1016/j.envres.2022.115165. ISSN 0013-9351. PMID 36584847. Bibcode: 2023ER....220k5165B.
- ↑ Arvaniti, Olga S.; Stasinakis, Athanasios S. (15 August 2015). "Review on the occurrence, fate and removal of perfluorinated compounds during wastewater treatment". Science of the Total Environment 524–525: 81–92. doi:10.1016/j.scitotenv.2015.04.023. PMID 25889547. Bibcode: 2015ScTEn.524...81A.
- ↑ US EPA, OW (September 1, 2015). "Third Unregulated Contaminant Monitoring Rule". https://www.epa.gov/dwucmr/third-unregulated-contaminant-monitoring-rule.
- ↑ "Biological monitoring of polyfluoroalkyl substances: A review". Environ. Sci. Technol. 40 (11): 3463–73. June 2006. doi:10.1021/es052580b. PMID 16786681. Bibcode: 2006EnST...40.3463H. Supporting Information (PDF).
- ↑ Dauwe, Tom; Van de Vijver, Kristin; De Coen, Wim; Eens, Marcel (April 2007). "PFOS levels in the blood and liver of a small insectivorous songbird near a fluorochemical plant". Environment International 33 (3): 357–361. doi:10.1016/j.envint.2006.11.014. PMID 17188355.
- ↑ Saini, Amandeep; Chinnadurai, Sita; Schuster, Jasmin K.; Eng, Anita; Harner, Tom (February 2023). "Per- and polyfluoroalkyl substances and volatile methyl siloxanes in global air: Spatial and temporal trends". Environmental Pollution 323: 121291. doi:10.1016/j.envpol.2023.121291. PMID 36796663.
- ↑ 41.0 41.1 Blake, Bevin E.; Fenton, Suzanne E. (October 2020). "Early life exposure to per- and polyfluoroalkyl substances (PFAS) and latent health outcomes: A review including the placenta as a target tissue and possible driver of peri- and postnatal effects". Toxicology 443: 152565. doi:10.1016/j.tox.2020.152565. ISSN 1879-3185. PMID 32861749.
- ↑ Gao, Xuping; Ni, Wanze; Zhu, Sui; Wu, Yanxin; Cui, Yunfeng; Ma, Junrong; Liu, Yanhua; Qiao, Jinlong et al. (October 2021). "Per- and polyfluoroalkyl substances exposure during pregnancy and adverse pregnancy and birth outcomes: A systematic review and meta-analysis". Environmental Research 201: 111632. doi:10.1016/j.envres.2021.111632. ISSN 1096-0953. PMID 34237336. Bibcode: 2021ER....201k1632G. https://pubmed.ncbi.nlm.nih.gov/34237336/.
- ↑ Stein, C. R.; Savitz, D. A.; Dougan, M. (19 August 2009). "Serum Levels of Perfluorooctanoic Acid and Perfluorooctane Sulfonate and Pregnancy Outcome". American Journal of Epidemiology 170 (7): 837–846. doi:10.1093/aje/kwp212. PMID 19692329.
- ↑ 44.0 44.1 44.2 44.3 "Health Effects Support Document for Perfluorooctane Sulfonate (PFOS)". https://www.epa.gov/sites/default/files/2016-05/documents/pfos_hesd_final_508.pdf.
- ↑ Gao, Xuping; Ni, Wanze; Zhu, Sui; Wu, Yanxin; Cui, Yunfeng; Ma, Junrong; Liu, Yanhua; Qiao, Jinlong et al. (October 2021). "Per- and polyfluoroalkyl substances exposure during pregnancy and adverse pregnancy and birth outcomes: A systematic review and meta-analysis". Environmental Research 201: 111632. doi:10.1016/j.envres.2021.111632. ISSN 1096-0953. PMID 34237336. Bibcode: 2021ER....201k1632G. https://pubmed.ncbi.nlm.nih.gov/34237336.
- ↑ Szilagyi, John T.; Freedman, Anastasia N.; Kepper, Stewart L.; Keshava, Arjun M.; Bangma, Jackie T.; Fry, Rebecca C. (2020-06-01). "Per- and Polyfluoroalkyl Substances Differentially Inhibit Placental Trophoblast Migration and Invasion In Vitro". Toxicological Sciences 175 (2): 210–219. doi:10.1093/toxsci/kfaa043. ISSN 1096-0929. PMID 32219433.
- ↑ Li, Xiaoheng; Ye, Leping; Ge, Yufei; Yuan, Kaiming; Zhang, Yufei; Liang, Yong; Wei, Jia; Zhao, Connie et al. (March 2016). "In utero perfluorooctane sulfonate exposure causes low body weights of fetal rats: A mechanism study". Placenta 39: 125–133. doi:10.1016/j.placenta.2016.01.010. ISSN 1532-3102. PMID 26992685. https://pubmed.ncbi.nlm.nih.gov/26992685/.
- ↑ Ames, Jennifer L.; Windham, Gayle C.; Lyall, Kristen; Pearl, Michelle; Kharrazi, Martin; Yoshida, Cathleen K.; Van de Water, Judy; Ashwood, Paul et al. (March 2020). "Neonatal Thyroid Stimulating Hormone and Subsequent Diagnosis of Autism Spectrum Disorders and Intellectual Disability". Autism Research 13 (3): 444–455. doi:10.1002/aur.2247. ISSN 1939-3806. PMID 31823519. https://pubmed.ncbi.nlm.nih.gov/31823519/.
- ↑ Moog, N.K.; Entringer, S.; Heim, C.; Wadhwa, P.D.; Kathmann, N.; Buss, C. (February 2017). "Influence of maternal thyroid hormones during gestation on fetal brain development". Neuroscience 342: 68–100. doi:10.1016/j.neuroscience.2015.09.070. ISSN 0306-4522. PMID 26434624. PMC 4819012. http://dx.doi.org/10.1016/j.neuroscience.2015.09.070.
- ↑ Mogensen, Ulla B.; Grandjean, Philippe; Nielsen, Flemming; Weihe, Pal; Budtz-Jørgensen, Esben (2015-09-01). "Breastfeeding as an Exposure Pathway for Perfluorinated Alkylates". Environmental Science & Technology 49 (17): 10466–10473. doi:10.1021/acs.est.5b02237. ISSN 1520-5851. PMID 26291735. Bibcode: 2015EnST...4910466M.
- ↑ Haug, Line S.; Huber, Sandra; Becher, Georg; Thomsen, Cathrine (May 2011). "Characterisation of human exposure pathways to perfluorinated compounds--comparing exposure estimates with biomarkers of exposure". Environment International 37 (4): 687–693. doi:10.1016/j.envint.2011.01.011. ISSN 1873-6750. PMID 21334069. https://pubmed.ncbi.nlm.nih.gov/21334069.
- ↑ "PFAS and Breastfeeding | ATSDR" (in en-us). 2021-11-19. https://www.atsdr.cdc.gov/pfas/health-effects/pfas-breastfeeding.html.
- ↑ "PFAS Chemicals: EDCs Contaminating Our Water and Food Supply" (in en). https://www.endocrine.org/topics/edc/what-edcs-are/common-edcs/pfas.
- ↑ Potera, Carol (April 2009). "REPRODUCTIVE TOXICOLOGY: Study Associates PFOS and PFOA with Impaired Fertility". Environmental Health Perspectives 117 (4): A148. doi:10.1289/ehp.117-a148a.
- ↑ Coperchini, F.; Awwad, O.; Rotondi, M.; Santini, F.; Imbriani, M.; Chiovato, L. (February 2017). "Thyroid disruption by perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA)". Journal of Endocrinological Investigation 40 (2): 105–121. doi:10.1007/s40618-016-0572-z. ISSN 1720-8386. PMID 27837466. https://pubmed.ncbi.nlm.nih.gov/27837466/.
- ↑ Dallaire, Renée; Dewailly, Éric; Pereg, Daria; Dery, Serge; Ayotte, Pierre (September 2009). "Thyroid Function and Plasma Concentrations of Polyhalogenated Compounds in Inuit Adults". Environmental Health Perspectives 117 (9): 1380–1386. doi:10.1289/ehp.0900633. PMID 19750101.
- ↑ Shahid, Muhammad A.; Ashraf, Muhammad A.; Sharma, Sandeep (2022), "Physiology, Thyroid Hormone", StatPearls (Treasure Island (FL): StatPearls Publishing), PMID 29763182, http://www.ncbi.nlm.nih.gov/books/NBK500006/, retrieved 2022-03-31
- ↑ Coperchini, Francesca; Croce, Laura; Ricci, Gianluca; Magri, Flavia; Rotondi, Mario; Imbriani, Marcello; Chiovato, Luca (2021). "Thyroid Disrupting Effects of Old and New Generation PFAS". Frontiers in Endocrinology 11: 612320. doi:10.3389/fendo.2020.612320. ISSN 1664-2392. PMID 33542707.
- ↑ Boesen, Sophie A. H.; Long, Manhai; Wielsøe, Maria; Mustieles, Vicente; Fernandez, Mariana F.; Bonefeld-Jørgensen, Eva C. (2020-10-13). "Exposure to Perflouroalkyl acids and foetal and maternal thyroid status: a review". Environmental Health 19 (1): 107. doi:10.1186/s12940-020-00647-1. ISSN 1476-069X. PMID 33050930.
- ↑ Steenland, K.; Tinker, S.; Frisbee, S.; Ducatman, A.; Vaccarino, V. (21 October 2009). "Association of Perfluorooctanoic Acid and Perfluorooctane Sulfonate With Serum Lipids Among Adults Living Near a Chemical Plant". American Journal of Epidemiology 170 (10): 1268–1278. doi:10.1093/aje/kwp279. PMID 19846564.
- ↑ Nelson, Jessica W.; Hatch, Elizabeth E.; Webster, Thomas F. (February 2010). "Exposure to Polyfluoroalkyl Chemicals and Cholesterol, Body Weight, and Insulin Resistance in the General U.S. Population". Environmental Health Perspectives 118 (2): 197–202. doi:10.1289/ehp.0901165. PMID 20123614.
- ↑ US EPA, OW (2016-05-05). "Drinking Water Health Advisories for PFOA and PFOS" (in en). https://www.epa.gov/ground-water-and-drinking-water/drinking-water-health-advisories-pfoa-and-pfos.
- ↑ Saikat, Sohel; Kreis, Irene; Davies, Bethan; Bridgman, Stephen; Kamanyire, Robie (February 2013). "The impact of PFOS on health in the general population: a review". Environmental Science: Processes & Impacts 15 (2): 329–335. doi:10.1039/c2em30698k. ISSN 2050-7895. PMID 25208696. https://pubmed.ncbi.nlm.nih.gov/25208696.
- ↑ Hoffman, Kate; Webster, Thomas F.; Weisskopf, Marc G.; Weinberg, Janice; Vieira, Verónica M. (December 2010). "Exposure to Polyfluoroalkyl Chemicals and Attention Deficit/Hyperactivity Disorder in U.S. Children 12–15 Years of Age". Environmental Health Perspectives 118 (12): 1762–1767. doi:10.1289/ehp.1001898. PMID 20551004.
- ↑ Liew, Zeyan; Ritz, Beate; von Ehrenstein, Ondine S.; Bech, Bodil Hammer; Nohr, Ellen Aagaard; Fei, Chunyuan; Bossi, Rossana; Henriksen, Tine Brink et al. (April 2015). "Attention deficit/hyperactivity disorder and childhood autism in association with prenatal exposure to perfluoroalkyl substances: a nested case-control study in the Danish National Birth Cohort". Environmental Health Perspectives 123 (4): 367–373. doi:10.1289/ehp.1408412. ISSN 1552-9924. PMID 25616253.
- ↑ 66.0 66.1 Shankar, Anoop; Jie Xiao; Alan Ducatman (2011-10-15). "Perfluoroalkyl Chemicals and Chronic Kidney Disease in US Adults". American Journal of Epidemiology 174 (8): 893–900. doi:10.1093/aje/kwr171. PMID 21873601.
- ↑ 67.0 67.1 OECD (2002). "Hazard Assessment of Perfluorooctane Sulfonate (PFOS) and its Salt". ENV/JM/RD(2002)17/FINAL (Page 5). https://www.oecd.org/env/ehs/risk-assessment/2382880.pdf.
- ↑ Zahm, Shelia; Bonde, Jens Peter; Chiu, Weihsueh A; Hoppin, Jane; Kanno, Jun; Abdallah, Mohamed; Blystone, Chad R; Calkins, Miriam M et al. (2023). "Carcinogenicity of perfluorooctanoic acid and perfluorooctanesulfonic acid". The Lancet Oncology. doi:10.1016/S1470-2045(23)00622-8.
- ↑ "PFAS Exposure and Risk of Cancer - National Cancer Institute" (in en). 2020-10-15. https://dceg.cancer.gov/research/what-we-study/pfas.
- ↑ Peden-Adams, M. M.; Keil, D. E.; Romano, T.; Mollenhauer, M. A. M.; Fort, D. J.; Guiney, P. D.; Houde, M.; Kannan, K. et al. (2009). "Health effects of perfluorinated compounds—What are the wildlife telling us?". Reproductive Toxicology 27 (3–4): 414–415. doi:10.1016/j.reprotox.2008.11.016.
- ↑ Peden-Adams et al. (June 2008). In PFAA Days II (PDF). p. 28.
- ↑ Peden-Adams, M.; Stuckey, J.; Gaworecki, K.; Berger-Ritchie, J.; Bryant, K.; Jodice, P.; Scott, T.; Ferrario, J. et al. (2009). "Developmental toxicity in white leghorn chickens following in ovo exposure to perfluorooctane sulfonate (PFOS)". Reproductive Toxicology (Elmsford, N.Y.) 27 (3–4): 307–318. doi:10.1016/j.reprotox.2008.10.009. PMID 19071210.
- ↑ SC-4/17: Listing of perfluorooctane sulfonic acid, its salts and perfluorooctane sulfonyl fluoride
- ↑ SC-9/4: Perfluorooctane sulfonic acid, its salts and perfluorooctane sulfonyl fluoride
- ↑ SCHER (2005). "RPA's report "Perfluorooctane Sulphonates Risk reduction strategy and analysis of advantages and drawbacks"". Scientific Committee on Health and Environmental Risks, European Commission.
- ↑ "DIRECTIVE 2006/122/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 12 December 2006". http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:372:0032:0034:EN:PDF.
- ↑ "COMMISSION REGULATION (EC) No 552/2009 of 22 June 2009". http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:164:0007:0031:EN:PDF.
- ↑ "COMMISSION REGULATION (EU) No 757/2010 of 24 August 2010". http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:223:0029:0036:EN:PDF.
- ↑ "Michigan abruptly sets PFAS cleanup rules" (in en). 2018-01-10. https://www.mlive.com/news/2018/01/michigan_pfos_pfoa_part_201_cr.html.
- ↑ Matheny, Keith (3 August 2020). "Michigan's drinking water standards for these chemicals now among toughest in nation". https://www.freep.com/story/news/local/michigan/2020/08/03/tougher-pfas-standards-drinking-water-michigan/5574268002/.
- ↑ "New state drinking water standards pave way for expansion of Michigan's PFAS clean-up efforts". 3 August 2020. https://www.michigan.gov/egle/0,9429,7-135--535602--,00.html.
- ↑ "Assembly Bill No. 2762". State of California. September 30, 2020. https://leginfo.legislature.ca.gov/faces/billTextClient.xhtml?bill_id=201920200AB2762.
- ↑ EPA (2021-03-03). "Announcement of Final Regulatory Determinations for Contaminants on the Fourth Drinking Water Contaminant Candidate List." Federal Register, 86 FR 12272
- ↑ RIN 2050-AH09
- ↑ Docket EPA-HQ-OLEM-2019-0341
External links
- Mason Chemical Company, Fluorosurfactant Structure/Function page
- PFOS risk assessment report
- Centers for Disease Control and Prevention, Polyfluorochemicals fact sheet
- Perfluorinated substances and their uses in Sweden
- Chain of Contamination: The Food Link, Perfluorinated Chemicals (PFCs) Incl. PFOS & PFOA
- Provisional evaluation of PFT in drinking water with the guide substances perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) as examples
 |


