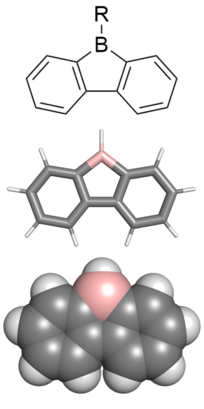
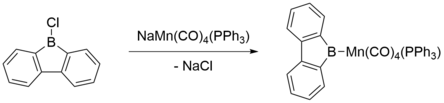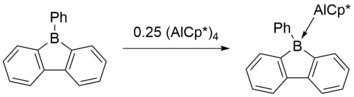Chemistry:9-Borafluorene
9-borafluorenes are a class of boron-containing heterocycles consisting of a tricyclic system with a central BC4 ring with two fused arene groups. 9-borafluorenes can be thought of as a borole with two fused arene rings, or as a trigonal planar boron atom with an empty p orbital bridging two biphenyl rings. However, 9-borafluorenes are generally less reactive than boroles due to less antiaromatic character and Lewis acidity. Containing highly conjugated π systems, 9-borafluorenes possess interesting photophysical properties. In addition, 9-borafluorenes are good Lewis acids. This combination of properties enables potential uses such as in light-emitting materials, solar cells, and sensors for some molecules.[1]
Synthesis
The earliest successful synthesis of a 9-borafluorene was reported in 1963 by Köster and Benedikt, who performed thermolysis of dialkyl- or diaryl-2-biphenylboranes to release an alkane and yield the 9-borafluorene. Treatment of the resulting 9-alkyl or 9-arylborafluorene with boron trichloride yields the 9-chloroborafluorene, which can be functionalized to a variety of derivatives.[2]
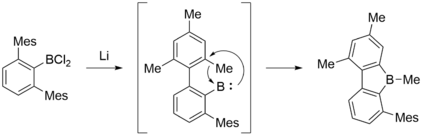
A particularly interesting synthesis of 9-borafluorenes was reported by Grigsby and Power. 2,6-Mes2C6H3BX2 (X = Cl, Br) was treated with lithium metal in diethyl ether to yield the reactive, highly electron-deficient borylene intermediate, which is able to insert into the strong C–C σ bond to form a 9-borafluorene.[3]
A highly useful synthetic route to 9-borafluorenes is transmetalation reactions that utilize a heteroatom in the 9-position. 9-mercurafluorenes,[4] 9-silafluorenes,[5] and 9-stannafluorenes[6] have been utilized in syntheses with generally good yield. While earlier synthetic methods often suffered from some substituents on the biphenyl framework leading to poor yield and selectivity, transmetalation methods generally tolerate substitution, giving rise to greater variety.
Functionalization
9-halo-9-borafluorenes are by far the most common precursors to functionalization of 9-borafluorenes at the boron center. The main strategies to accomplish late-stage functionalization at the boron center are metal halide elimination reactions using organometallic reagents, trialkylsilyl halide elimination, and hydrogen halide elimination using a base and either an amine or alcohol.[1]
Reactivity
Lewis acid-base adducts
9-borafluorenes are highly Lewis acidic at the boron center and readily form Lewis acid-base adducts to satisfy the octet for the boron atom. In these adducts, the boron center is no longer trigonal planar and no longer has its empty p orbital that participates in conjugation in the π system in 9-borafluorene. Adducts involving Lewis bases such as pyridines,[7] phosphines,[8] ethers,[9] carbenes,[10] and nitriles[11] have been described. The reactions involve simple reaction of the 9-borafluorene with the Lewis base at room temperature or low temperature in moderate to high yield. In addition, 9-borafluorenes whose boron is substituted with a group that also contains a Lewis base, such as 8-hydroxyquinioline,[12] can form intramolecular adducts via the substituent's Lewis base donating to the boron atom to form a boron spirocenter.
Reduction
The 4π antiaromatic BC4 ring can undergo two-electron reduction to form a 6π aromatic system to form a dianion.
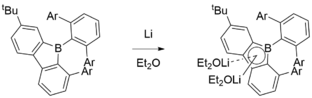
This can be accomplished by reduction by lithium metal. X-ray diffraction studies show that upon reduction, the B–C bond shortens from 1.64 Å to 1.54 Å owing to the gain of aromaticity.[13] One-electron reduction of 9-bromo-9-borafluorene NHC and CAAC adducts has also yielded isolable neutral 9-borafluorene radicals.[14]
B–C bond insertion
The endocyclic B–C bond in 9-borafluorenes is often susceptible to insertion by a variety of reagents.


Alkynes and phosphaalkynes have been shown to insert into the B–C bond to yield a 7-membered ring system. Mechanistic calculations revealed that the reaction between diphenylacetylene and 9-chloro-9-borafluorene occurs first by coordination of the alkyne π bond to the boron center, followed by insertion into the B–C bond in a concerted step with a single transition state to yield the BC6 system.[15] In contrast, mechanistic calculations indicate that the reaction of 1-adamantyl-phosphaalkyne and 9-phenyl-9-borafluorene occurs via a concerted transition state.[16]
Carbenes have also been shown to perform insertion. Bartholeme, Bluer, and Martin reacted 9-phenyl-9-borafluorene with CH(TMS)=N2 which generates a carbene via loss of N2. Insertion generated a BC5 system, which could then undergo a subsequent insertion with another equivalent of the carbene to yield a symmetric species with a BC6 system.
In addition to alkynes, phosphaalkynes, and carbenes, insertion reactions into 9-borafluorenes have been shown with other functional groups such as azides[17] and carbonyls.[11]
Metal complexes
Transition metal complexes involving 9-borafluorenes include those in which the 9-borafluorene acts as an L-type ligand similar to a metal-boryl complex. These were the earliest 9-borafluorene complexes to be synthesized, and usually involved reaction of 9-chloro-9-borafluorene with alkali metal salts of anionic transition metal complexes, such as Mn(-I).[18] Upon reaction, the alkali metal chloride is eliminated and the transition metal undergoes two-electron oxidation to yield the metal-boryl complex. Such complexes are essentially metal-boryl complexes, in which the 9-borafluorene ligand acts as a σ donor and π acceptor.[19] (Co(II), and Co(III) complexes of this type have also been synthesized.[20][21])
η1 complexes in which the 9-borafluorene acts as a Z-type ligand accepting the metal center's electrons into the empty boron p orbital have been synthesized, including the complex between 9-alkyl- or 9-aryl-9-borafluorenes and (pentamethylcyclopentadienyl)aluminium(I) (AlCp*) in which the aluminium center donates into the empty boron p orbital.[6]
Attempts to synthesize η5 9-borafluorene complexes with aluminium(III) were unsuccessful, but an η5 complex with Ni(0) was synthesized by Harman et al. by reaction of a phosphine-appended 9-borafluorene ligand with tetrakis(triphenylphosphine)nickel. X-ray diffraction studies of the resulting product showed Ni–C distances that indicated interaction between the nickel atom and the carbon atoms in the central BC4 ring. DFT calculations led the authors to describe the borafluorene complex as an L2 ligand with significant nickel backbonding into the empty boron p orbital.[22]
Oligomerizations
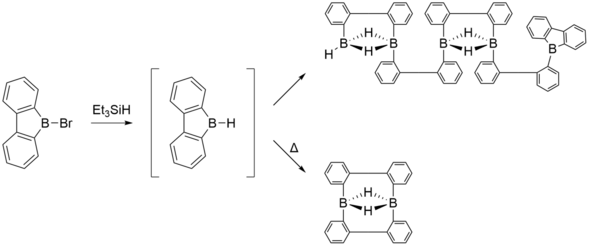
9-borafluorenes can participate in ring-opening reactions to form oligomers, which often contain three-center two-electron bonds, in order to fulfill the octet on the boron atom. For example, it has been reported that a 1:1 mixture of 9-bromo-9-borafluorene and triethylsilane at room temperature for several weeks led to a pentameric species as a result of a ring-opening reaction. However, the same reaction under high temperature yielded the dimer.[23]
Properties
Photophysical properties
The presence of π-conjugation throughout the biphenyl unit and the empty p orbital of 9-borafluorenes leads to interesting properties such as fluorescence. For example, IPr ((HCNDipp)2C:), IPrCH2 ((HCNDipp)2C=CH2), PCy3, and PPh3 monoadducts of 9-bromo-9-borafluorene displayed blue emission peaks at λ=435 nm, suggesting that the fluorescence arises from the 9-borafluorene scaffold and that the identity of the Lewis base coordinating the boron center does not alter the fluorescent properties.[8] However, 9-borafluorenes with substituents possessing an additional Lewis basic functional group, such as 8-hydroxyquinoline, show higher quantum yield due to increased rigidity of the molecule.[12] A similar phenomenon was observed with BODIPY and aza-BODIPY coordinating to the boron center, where the HOMO-LUMO gaps of each π system were relatively unchanged, but increased rigidity led to improved quantum yield.[24]
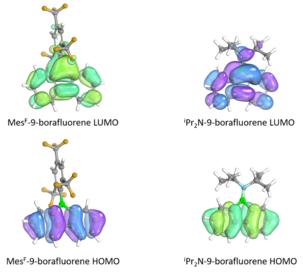
While the identity of the Lewis base in the 9-borafluorene adduct does not affect the emission, the identity of the substituent at the boron center have been found to affect photophysical properties. π donor groups such as tBuO and iPr2N were found to blue-shift the absorption peak attributed to raising the energy of the LUMO, while electron acceptor groups such as MesF (2,4,6-(tris(trifluoromethyl))phenyl) were found to red-shift the absorption by lowering the energy of the LUMO. Computational studies have been performed and have calculated that the while the HOMOs of the MesF and iPr2N substituted 9-borafluorenes have energies -6.37 and -5.85 eV, the LUMOs have energies -2.77 and -1.68 eV. The bonding interaction of MesF and the antibonding interaction of iPr2N with the LUMO are visualized with the frontier molecular orbitals above, explaining the trends in LUMO energy. The HOMO has a small contribution from the boron atom and is thus affected relatively little by the substituent.[25]
Sensors
Though the identity of the Lewis base in adducts does not affect emission, the change in hybridization of the boron center upon adduct formation alters the π system of 9-borafluorenes. The combination of the photophysical properties and Lewis acidities enables 9-borafluorenes to have potential applications as molecular sensors.
Yamaguchi et al. investigated an early 9-borafluorene sensor for fluoride ion using fluorescent properties, observing that THF solutions of the sensor possessed an emission maximum of around 560 nm, whereas upon addition of fluoride ion using TBAF the 560 nm peak disappeared and an emission maximum around 420 nm appeared. In addition, adding a strong fluoride scavenger such as BF3•OEt2 reversed the changes. The sensing was attributed to the binding of fluoride ion increasing the HOMO-LUMO gap by changing the pπ-π* conjugation.[26]
Another sensor, an NHC-stabilized 3-methoxy-9-borafluorenium cation, displays thermochromism based on intermolecular coordination of the oxygen atom in the methoxy group of one molecule to a boron center in another molecule. It was observed that a solution of the sensor in a weakly coordinating solvent was red at room temperature but became colorless upon cooling.[10]
A variety of other 9-borafluorene-based sensors have been developed, including those using 9-borafluorene copolymers, those sensing species such as ammonia, and those sensing alkane solvent chain length by utilizing solvatochromism.[1]
References
- ↑ 1.0 1.1 1.2 Su, Xiaojun; Bartholome, Tyler A.; Tidwell, John R.; Pujol, Alba; Yruegas, Sam; Martinez, Jesse J.; Martin, Caleb D. (2021-03-03). "9-Borafluorenes: Synthesis, Properties, and Reactivity". Chemical Reviews 121 (7): 4147–4192. doi:10.1021/acs.chemrev.0c01068. PMID 33656339. https://pubs.acs.org/doi/pdf/10.1021/acs.chemrev.0c01068.
- ↑ Köster, R.; Benedikt, G. (1963). "9-Borafluorenes" (in en). Angewandte Chemie International Edition in English 2 (6): 323–324. doi:10.1002/anie.196303232. https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.196303232.
- ↑ Grigsby, Warren J.; Power, Philip P. (1996-01-01). "Isolation and Reduction of Sterically Encumbered Arylboron Dihalides: Novel Boranediyl Insertion into C−C σ-Bonds". Journal of the American Chemical Society 118 (34): 7981–7988. doi:10.1021/ja960918j. https://pubs.acs.org/doi/pdf/10.1021/ja960918j.
- ↑ Narula, Chaitanya K.; Nöth, Heinrich (1985-02-19). "Contributions to the chemistry of boron: CLVI. A convenient route to 9-borafluorenes" (in en). Journal of Organometallic Chemistry 281 (2): 131–134. doi:10.1016/0022-328X(85)87101-1. https://dx.doi.org/10.1016/0022-328X%2885%2987101-1.
- ↑ Gross, Ulrich; Kaufmann, Dieter (1987). "Borylierung von Arylsilanen, III Reaktionen silylierter Biphenyle und 9H-9-Silafluorene mit Tribromboran" (in de). Chemische Berichte 120 (6): 991–994. doi:10.1002/cber.19871200617. https://onlinelibrary.wiley.com/doi/abs/10.1002/cber.19871200617.
- ↑ 6.0 6.1 Romero, Patricio E.; Piers, Warren E.; Decker, Stephen A.; Chau, Dan; Woo, Tom K.; Parvez, Masood (2003-03-01). "η1 versus η5 Bonding Modes in Cp*Al(I) Adducts of 9-Borafluorenes". Organometallics 22 (6): 1266–1274. doi:10.1021/om0209935. https://doi.org/10.1021/om0209935.
- ↑ Narula, Chaitanya K.; Noeth, Heinrich (1985-07-01). "Contribution to the chemistry of boron. 150. Competition between adduct and cation formation in reactions between diorganylborane derivatives and pyridine or lutidines". Inorganic Chemistry 24 (16): 2532–2539. doi:10.1021/ic00210a014. https://doi.org/10.1021/ic00210a014.
- ↑ 8.0 8.1 Berger, Christopher J.; He, Gang; Merten, Christian; McDonald, Robert; Ferguson, Michael J.; Rivard, Eric (2014-02-03). "Synthesis and Luminescent Properties of Lewis Base-Appended Borafluorenes". Inorganic Chemistry 53 (3): 1475–1486. doi:10.1021/ic402408t. PMID 24428809. https://doi.org/10.1021/ic402408t.
- ↑ Zhang, Weidong; Yu, Demei; Wang, Zhijun; Zhang, Bingjie; Xu, Letian; Li, Guoping; Yan, Ni; Rivard, Eric et al. (2019-01-04). "Dibora[10annulenes: Construction, Properties, and Their Ring-Opening Reactions"]. Organic Letters 21 (1): 109–113. doi:10.1021/acs.orglett.8b03538. PMID 30560667. https://doi.org/10.1021/acs.orglett.8b03538.
- ↑ 10.0 10.1 Yang, Wenlong; Krantz, Kelsie E.; Freeman, Lucas A.; Dickie, Diane A.; Molino, Andrew; Kaur, Aishvaryadeep; Wilson, David J. D.; Gilliard, Robert J. (2019). "Stable Borepinium and Borafluorenium Heterocycles: A Reversible Thermochromic "Switch" Based on Boron–Oxygen Interactions" (in en). Chemistry – A European Journal 25 (54): 12512–12516. doi:10.1002/chem.201903348. PMID 31334883. https://onlinelibrary.wiley.com/doi/abs/10.1002/chem.201903348.
- ↑ 11.0 11.1 Bluer, Kristen R.; Laperriere, Leif E.; Pujol, Alba; Yruegas, Sam; Adiraju, Venkata A. K.; Martin, Caleb D. (2018-09-10). "Coordination and Ring Expansion of 1,2-Dipolar Molecules with 9-Phenyl-9-borafluorene". Organometallics 37 (17): 2917–2927. doi:10.1021/acs.organomet.8b00497. https://doi.org/10.1021/acs.organomet.8b00497.
- ↑ 12.0 12.1 Urban, Mateusz; Durka, Krzysztof; Górka, Patrycja; Wiosna-Sałyga, Gabriela; Nawara, Krzysztof; Jankowski, Piotr; Luliński, Sergiusz (2019-06-18). "The effect of locking π-conjugation in organoboron moieties in the structures of luminescent tetracoordinate boron complexes" (in en). Dalton Transactions 48 (24): 8642–8663. doi:10.1039/C9DT01332F. PMID 31123739. https://pubs.rsc.org/en/content/articlelanding/2019/dt/c9dt01332f.
- ↑ 13.0 13.1 Wehmschulte, Rudolf J.; Khan, Masood A.; Twamley, Brendan; Schiemenz, Berthold (2001-03-01). "Synthesis and Characterization of a Sterically Encumbered Unsymmetrical 9-Borafluorene, Its Pyridine Adduct, and Its Dilithium Salt". Organometallics 20 (5): 844–849. doi:10.1021/om000868y. https://doi.org/10.1021/om000868y.
- ↑ Yang, Wenlong; Krantz, Kelsie E.; Freeman, Lucas A.; Dickie, Diane A.; Molino, Andrew; Frenking, Gernot; Pan, Sudip; Wilson, David J. D. et al. (2020). "Persistent Borafluorene Radicals" (in en). Angewandte Chemie International Edition 59 (10): 3850–3854. doi:10.1002/anie.201909627. PMID 31816143.
- ↑ 15.0 15.1 Shoji, Yoshiaki; Shigeno, Naoki; Takenouchi, Kumiko; Sugimoto, Manabu; Fukushima, Takanori (2018). "Mechanistic Study of Highly Efficient Direct 1,2-Carboboration of Alkynes with 9-Borafluorenes" (in en). Chemistry – A European Journal 24 (50): 13223–13230. doi:10.1002/chem.201801818. PMID 29923245. https://onlinelibrary.wiley.com/doi/abs/10.1002/chem.201801818.
- ↑ Yruegas, Sam; Barnard, Jonathan H.; Al-Furaiji, Khalidah; Dutton, Jason L.; Wilson, David J. D.; Martin, Caleb D. (2018-05-29). "Boraphosphaalkene Synthesis via Phosphaalkyne Insertion into 9-Borafluorene". Organometallics 37 (10): 1515–1518. doi:10.1021/acs.organomet.8b00248. https://doi.org/10.1021/acs.organomet.8b00248.
- ↑ Yruegas, Sam; Martinez, Jesse J.; Martin, Caleb D. (2018-06-19). "Intermolecular insertion reactions of azides into 9-borafluorenes to generate 9,10-B,N-phenanthrenes" (in en). Chemical Communications 54 (50): 6808–6811. doi:10.1039/C8CC01529E. PMID 29547215. https://pubs.rsc.org/en/content/articlelanding/2018/cc/c8cc01529e.
- ↑ Nöth, H.; Schmid, G. (1966). "Metall–Bor-Verbindungen. III. Über Triphenylphosphin-tetracarbonylmangan–Bor-Verbindungen" (in de). Zeitschrift für anorganische und allgemeine Chemie 345 (1–2): 69–78. doi:10.1002/zaac.19663450109. https://onlinelibrary.wiley.com/doi/abs/10.1002/zaac.19663450109.
- ↑ Irvine, Geoffrey J.; Lesley, M. J. Gerald; Marder, Todd B.; Norman, Nicholas C.; Rice, Craig R.; Robins, Edward G.; Roper, Warren R.; Whittell, George R. et al. (1998-11-21). "Transition Metal−Boryl Compounds: Synthesis, Reactivity, and Structure". Chemical Reviews 98 (8): 2685–2722. doi:10.1021/cr9500085. PMID 11848976. https://pubs.acs.org/doi/pdf/10.1021/cr9500085.
- ↑ Schmid, Günter; Nöth, Heinrich (1967). "Metall-Bor-Verbindungen, V. Die Diarylbor-Gruppe als Ligand in Phosphin-kobalt(II)-Verbindungen" (in de). Chemische Berichte 100 (9): 2899–2907. doi:10.1002/cber.19671000914. https://onlinelibrary.wiley.com/doi/abs/10.1002/cber.19671000914.
- ↑ Schmid, Günter; Powell, Paul; Nöth, Heinrich (1968). "Metall-Bor-Verbindungen, VI. Bis(dimethylglyoximato)-kobalt(III)-Verbindungen mit Co—B-Bindungen" (in de). Chemische Berichte 101 (4): 1205–1214. doi:10.1002/cber.19681010412. https://onlinelibrary.wiley.com/doi/abs/10.1002/cber.19681010412.
- ↑ Essex, Laura A.; Taylor, Jordan W.; Harman, W. Hill (2019-04-12). "Nickel complexes of phosphine-appended benzannulated boron heterocycles" (in en). Tetrahedron 75 (15): 2255–2260. doi:10.1016/j.tet.2019.02.047. https://www.sciencedirect.com/science/article/pii/S0040402019302078.
- ↑ Hübner, Alexander; Qu, Zheng-Wang; Englert, Ulli; Bolte, Michael; Lerner, Hans-Wolfram; Holthausen, Max C.; Wagner, Matthias (2011-03-02). "Main-Chain Boron-Containing Oligophenylenes via Ring-Opening Polymerization of 9-H-9-Borafluorene". Journal of the American Chemical Society 133 (12): 4596–4609. doi:10.1021/ja110947k. PMID 21366256. https://pubs.acs.org/doi/pdf/10.1021/ja110947k.
- ↑ Bonnier, Catherine; Piers, Warren E.; Al-Sheikh Ali, Adeeb; Thompson, Alison; Parvez, Masood (2009-08-24). "Perfluoroaryl-Substituted Boron Dipyrrinato Complexes". Organometallics 28 (16): 4845–4851. doi:10.1021/om900402e. https://doi.org/10.1021/om900402e.
- ↑ Smith, Mallory F.; Cassidy, S. Joel; Adams, Ian A.; Vasiliu, Monica; Gerlach, Deidra L.; Dixon, David A.; Rupar, Paul A. (2016-09-26). "Substituent Effects on the Properties of Borafluorenes". Organometallics 35 (18): 3182–3191. doi:10.1021/acs.organomet.6b00537. https://doi.org/10.1021/acs.organomet.6b00537.
- ↑ Yamaguchi, Shigehiro; Shirasaka, Toshiaki; Akiyama, Seiji; Tamao, Kohei (2002-07-01). "Dibenzoborole-Containing π-Electron Systems: Remarkable Fluorescence Change Based on the "On/Off" Control of the pπ−π* Conjugation". Journal of the American Chemical Society 124 (30): 8816–8817. doi:10.1021/ja026689k. PMID 12137533. https://doi.org/10.1021/ja026689k.
 |