Chemistry:ADX-71149
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
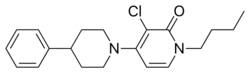
| Formula | C20H25ClN2O |
| Molar mass | 344.88 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
ADX-71149, also known as JNJ-40411813 and JNJ-mGluR2-PAM, is a selective positive allosteric modulator of the mGlu2 receptor.[1][2][3] It is being studied by Addex Therapeutics and Janssen Pharmaceuticals for the treatment of schizophrenia.[4] It was also researched by these companies for the treatment of anxious depression (major depressive disorder with anxiety symptoms),[5] but although some efficacy was observed in clinical trials, it was not enough to warrant further development for this indication.[6] (As of 2015), ADX-71149 is in phase II clinical trials for schizophrenia.[4]
See also
- Biphenylindanone A
- Eglumegad
- LY-404,039
- LY-379,268
- LY-487,379
References
- ↑ "Discovery of 1-butyl-3-chloro-4-(4-phenyl-1-piperidinyl)-(1H)-pyridone (JNJ-40411813): a novel positive allosteric modulator of the metabotropic glutamate 2 receptor". Journal of Medicinal Chemistry 57 (15): 6495–512. August 2014. doi:10.1021/jm500496m. PMID 25032784.
- ↑ "Pharmacological and pharmacokinetic properties of JNJ-40411813, a positive allosteric modulator of the mGlu2 receptor". Pharmacology Research & Perspectives 3 (1): e00096. February 2015. doi:10.1002/prp2.96. PMID 25692015.
- ↑ "Preclinical evaluation of the antipsychotic potential of the mGlu2-positive allosteric modulator JNJ-40411813". Pharmacology Research & Perspectives 3 (2): e00097. March 2015. doi:10.1002/prp2.97. PMID 25692027.
- ↑ 4.0 4.1 "Group I and group II metabotropic glutamate receptor allosteric modulators as novel potential antipsychotics". Current Opinion in Pharmacology 20: 40–5. February 2015. doi:10.1016/j.coph.2014.11.003. PMID 25462291.
- ↑ "Schizophrenia drug discovery and development in an evolving era: are new drug targets fulfilling expectations?". Journal of Psychopharmacology 29 (2): 230–8. February 2015. doi:10.1177/0269881114565806. PMID 25586401.
- ↑ Addex Therapeutics (7 February 2014). "Addex Reports Top-line Data from ADX71149 Phase 2a Study in Patients with Major Depressive Disorder (MDD) with Significant Anxiety Symptoms". http://www.addextherapeutics.com/investors/press-releases/news-details/article/addex-reports-top-line-data-from-adx71149-phase-2a-study-in-patients-with-major-depressive-disorder/.
External links
- ADX71149 for Schizophrenia - Addex Therapeutics
- ADX71149 for Anxiety - Addex Therapeutics
- ADX 71149 - AdisInsight
 |

