Chemistry:Acetaldehyde ammonia trimer
From HandWiki
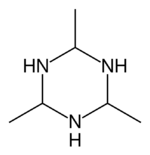
| |
| Names | |
|---|---|
| Preferred IUPAC name
2,4,6-Trimethyl-1,3,5-triazinane | |
| Other names
Acetaldehyde ammonia trimer
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C6H15N3 | |
| Molar mass | 129.207 g·mol−1 |
| Appearance | Colorless crystals |
| Melting point | 95 to 97 °C (203 to 207 °F; 368 to 370 K) |
| Solubility | polar organic solvents |
| Hazards | |
| GHS pictograms | 
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Acetaldehyde ammonia trimer is a chemical compound described by the formula (CH3CHNH)3. The pure material is colourless but samples often appear light yellow or slightly beige due to the degradation by oxidation. It is hygroscopic, and can be found in a trihydrate form.
As implied by its name, it is a trimeric species formed from the reaction of acetaldehyde and ammonia:
- 3 CH3CHO + 3 NH3 → (CH3CHNH)3 + 3 H2O
Studies using NMR spectroscopy indicate that the three methyl groups are equatorial, thus the molecule has C3v point group symmetry.[2]
The compound is related to hexamethylenetetramine, which is the condensation product of ammonia and formaldehyde.
References
- ↑ GHS: PubChem 69486
- ↑ Nielsen, A. T.; Atkins, R. L.; Moore, D. W.; Scott, R.; Mallory, D.; LaBerge, J. M. (1973). "Structure and Chemistry of the Aldehyde Ammonias. 1-Amino-1-alkanols, 2,4,6-Trialkyl-1,3,5-hexahydrotriazines, and N,N-Dialkylidene-1,1-Diaminoalkanes". Journal of Organic Chemistry 38 (19): 3288–3295. doi:10.1021/jo00959a010.
External links
 |

