Chemistry:Acetoguanamine
From HandWiki
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
6-Methyl-1,3,5-triazine-2,4-diamine[1] | |
| Other names
Diamino-6-methyl-1,3,5-triazine[citation needed]
| |
| Identifiers | |
3D model (JSmol)
|
|
| 118348 | |
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C4H7N5 | |
| Molar mass | 125.135 g·mol−1 |
| Appearance | White, opaque crystals |
| Density | 1.391 g cm−3 |
| Melting point | 274 to 276 °C (525 to 529 °F; 547 to 549 K) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P305+351+338 | |
| NFPA 704 (fire diamond) | |
| Flash point | 252 °C (486 °F; 525 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
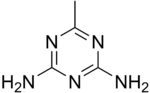
Acetoguanamine is an organic compound with the chemical formula (CNH2)2CCH3N3. It is related to melamine but with one amino group replaced by methyl. Acetoguanamine is used in the manufacturing of melamine resins. Unlike melamine ((CNH2)3N3), acetoguanamine is not a crosslinker. The "aceto" prefix is historical, the compound does not contain an acetyl group. A related compound is benzoguanamine.[2]
The compound is prepared by condensation of cyanoguanidine with acetonitrile:
- (H2N)2C=NCN + MeCN → (CNH2)2(CMe)N3
Safety
LD50 (oral, rats) is 2740 mg/kg.
References
- ↑ "Acetoguanamine - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology information. https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=10949.
- ↑ H. Deim; G. Matthias; R. A. Wagner (2012). "Amino Resins". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_115.pub2. ISBN 978-3527306732.
 |

