Chemistry:Acetolactic acid
From HandWiki
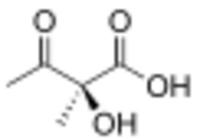
| |
| Names | |
|---|---|
| Preferred IUPAC name
(2S)-2-Hydroxy-2-methyl-3-oxobutanoic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C5H8O4 | |
| Molar mass | 132.115 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
α-Acetolactic acid is a precursor in the biosynthesis of the branched chain amino acids valine and leucine. α-Acetolactic acid is produced from two molecules of pyruvic acid by acetolactate synthase. α-Acetolactic acid can also be decarboxylated by alpha-acetolactate decarboxylase to produce acetoin.[1][2] The name α-acetolactate is used for anion (conjugate base), salts, and esters of α-acetolactic acid.
References
- ↑ Wood, B. J. B.; Holzapfel, W. H. (1995). "Carbohydrate Metabolism". The Lactic Acid Bacteria: The genera of lactic acid bacteria. 2. Springer. pp. 185–186. ISBN 978-0-7514-0215-5. https://books.google.com/books?id=Q8B_WusVacsC&pg=PA185.
- ↑ Marth, E. H.; Steele, J. L. (2001). "Genetics of Lactic acid bacteria". Applied dairy microbiology. 110 of Food science and technology. A series of monographs. CRC Press. pp. 283. ISBN 978-0-8247-0536-7. https://books.google.com/books?id=Sabnh9l76W0C&pg=PA283.
 |

