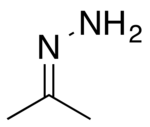
Chemistry:Acetone hydrazone
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
Propan-2-ylidenehydrazine
| |
| Other names
Isopropylidenehydrazine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C3H8N2 | |
| Molar mass | 72.111 g·mol−1 |
| Hazards | |
| GHS pictograms |     
|
| GHS Signal word | Danger |
| H226, H301, H311, H314, H331, H351, H411 | |
| P203Script error: No such module "Preview warning".Category:GHS errors, P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P273, P280, P301+316Script error: No such module "Preview warning".Category:GHS errors, P301+330+331, P302+352, P302+361+354Script error: No such module "Preview warning".Category:GHS errors, P303+361+353, P304+340, P305+354+338Script error: No such module "Preview warning".Category:GHS errors, P316Script error: No such module "Preview warning".Category:GHS errors, P318Script error: No such module "Preview warning".Category:GHS errors, P321, P330 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
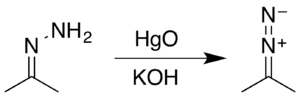
Acetone hydrazone (isopropylidenehydrazine) is the product of condensation of acetone and hydrazine, as typical for hydrazone formation. It is an intermediate in the synthesis of 2-diazopropane (fr).[2]
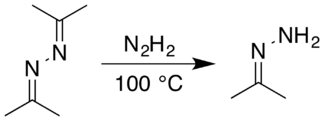
Acetone hydrazone can be produced on large scale by reaction of acetone azine with hydrazine, a more convenient reaction than direct reaction of acetone and hydrazine.[3] Likewise, it is susceptible to disproportionation to revert to acetone azine and hydrazine, especially in the presence of water.[3]
The chemical is one of the metabolic products of the antihypertensive pharmaceutical hydralazine, and itself also have antihypertensive effects.[4]
References
- ↑ "Acetone hydrazone" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/78937#section=Safety-and-Hazards.
- ↑ Andrews, S. D.; Day, A. C.; Raymond, P.; Whiting, M. C. (1970). "2-Diazopropane". Organic Syntheses: 27. http://www.orgsyn.org/demo.aspx?prep=cv6p0392.; Collective Volume, 6, 1988, pp. 392
- ↑ 3.0 3.1 Day, A. C.; Whiting, M. C.. "Acetone Hydrazone". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=cv6p0010.; Collective Volume, 6, pp. 10
- ↑ "Direct-acting vasodilators". Journal of Clinical Hypertension 13 (9): 690–692. 2011. doi:10.1111/j.1751-7176.2011.00507.x. PMID 21896152.
 |