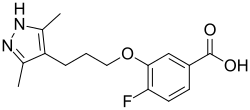
Chemistry:Acoramidis
 | |
| Clinical data | |
|---|---|
| Pronunciation | ə-corAM-i-dis |
| Trade names | Attruby, others |
| Other names | AG10 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a625013 |
| License data |
|
| Routes of administration | By mouth |
| Drug class | Amyloidogenesis suppressant |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
| Formula | C15H17FN2O3 |
| Molar mass | 292.310 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Acoramidis, sold under the brand name Attruby, is a medication used for the treatment of cardiomyopathy.[1] It is a near-complete (>90%) transthyretin stabilizer, developed to mimic the protective properties of the naturally occurring T119M mutation,[4][5] to treat transthyretin amyloid cardiomyopathy. It is taken by mouth.[1]
The most common adverse reactions include diarrhea and upper abdominal pain.[6]
Acoramidis was approved for medical use in the United States in November 2024,[6][7][8] and in the European Union in February 2025.[2][3]
Medical uses
Acoramidis is indicated for the treatment of the cardiomyopathy of wild-type or variant transthyretin-mediated amyloidosis (ATTR-CM) in adults to reduce cardiovascular death and cardiovascular-related hospitalization.[1][6][9]
Side effects
The most common side effects are diarrhea and abdominal pain.[10]
History
The efficacy and safety of acoramidis were evaluated in a multicenter, international, randomized, double-blind, placebo-controlled study in 611 adult participants with wild-type or hereditary (variant) ATTR-CM (NCT03860935).[6]
Clinical trials
Phase I data indicated acoramidis achieved near-complete (>90%) TTR stabilization across the entire dosing interval at steady state.[11]
Phase II and the Open-Label Extension (OLE) data indicated after a median of 38 months, long-term treatment with acoramidis was generally well tolerated and resulted in a median decline in NT-proBNP levels, normalization of serum TTR, and sustained stabilization of TTR in individuals with ATTR-CM. [12]
Phase III data from ATTRibute-CM indicated acoramidis resulted in a significantly better four-step primary hierarchical outcome containing components of mortality, morbidity, and function than placebo at 30 months in participants with ATTR-CM. Adverse events were similar in the two groups.[13]
Other analyses from ATTRibute-CM indicated a 50% reduction in cumulative cardiovascular hospitalizations (CVH), a 42% reduction in all-cause mortality (ACM) and recurrent CVH, and a 3-month time-to-separation of the Kaplan Meier curves for ACM or CVH. No other treatment has demonstrated this degree of treatment effect this quickly in participants with ATTR-CM.[14][15][16]
In vitro data indicated acoramidis exhibits near-complete (>90%) TTR stabilization at therapeutic trough concentrations, and its TTR stabilization exceeds that of tafamidis' across a range of destabilizing TTR mutations.[17]
Society and culture
Legal status
Acoramidis was approved for medical use in the United States in November 2024.[6][7][18] The approval was granted to BridgeBio Pharma.[9]
In December 2024, the Committee for Medicinal Products for Human Use of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Beyonttra, intended for the treatment of transthyretin amyloidosis in adults with cardiomyopathy.[2] The applicant for this medicinal product is BridgeBio Europe B.V.[2] Acoramidis was designated an orphan medicine by the EMA.[2] Acoramidis was authorized for medical use in the European Union in February 2025.[2][3]
Names
During development, acoramidis was known as AG10 (the Alhamadsheh-Graef molecule 10).[19]
Acoramidis is the international nonproprietary name.[20]
Acoramidis is sold under the brand names Attruby[1][6] and Beyonttra.[2][3]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "Attruby- acoramidis hydrochloride tablet, film coated". 26 November 2024. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=913552ef-875d-4cb7-bf05-a7d20a394c38.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 "Beyonttra EPAR". 12 December 2024. https://www.ema.europa.eu/en/medicines/human/EPAR/beyonttra. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ 3.0 3.1 3.2 3.3 "Beyonttra PI". 11 February 2025. https://ec.europa.eu/health/documents/community-register/html/h1906.htm.
- ↑ "AG10 inhibits amyloidogenesis and cellular toxicity of the familial amyloid cardiomyopathy-associated V122I transthyretin". Proceedings of the National Academy of Sciences of the United States of America 110 (24): 9992–9997. June 2013. doi:10.1073/pnas.1300761110. PMID 23716704. Bibcode: 2013PNAS..110.9992P.
- ↑ "Enthalpy-Driven Stabilization of Transthyretin by AG10 Mimics a Naturally Occurring Genetic Variant That Protects from Transthyretin Amyloidosis". Journal of Medicinal Chemistry 61 (17): 7862–7876. September 2018. doi:10.1021/acs.jmedchem.8b00817. PMID 30133284.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 "FDA approves drug for heart disorder caused by transthyretin-mediated". 1 October 2024. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-drug-heart-disorder-caused-transthyretin-mediated-amyloidosis.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 7.0 7.1 "Novel Drug Approvals for 2024". 1 October 2024. https://www.fda.gov/drugs/novel-drug-approvals-fda/novel-drug-approvals-2024.
- ↑ (PDF) New Drug Therapy Approvals 2024 (Report). January 2025. https://www.fda.gov/media/184967/download. Retrieved 21 January 2025.
- ↑ 9.0 9.1 "Bridgebio's Attruby, to Treat Heart Condition ATTR-CM, Receives FDA Approval". 25 November 2024. https://www.genengnews.com/topics/drug-discovery/bridgebios-attruby-to-treat-heart-condition-attr-cm-receives-fda-approval/.
- ↑ "FDA approves BridgeBio's Attruby for ATTR-CM treatment". 25 November 2024. https://www.pharmaceutical-technology.com/news/fda-bridgebios-attruby-attr-cm/.
- ↑ "First-in-Human Study of AG10, a Novel, Oral, Specific, Selective, and Potent Transthyretin Stabilizer for the Treatment of Transthyretin Amyloidosis: A Phase 1 Safety, Tolerability, Pharmacokinetic, and Pharmacodynamic Study in Healthy Adult Volunteers". Clinical Pharmacology in Drug Development 9 (1): 115–129. January 2020. doi:10.1002/cpdd.700. PMID 31172685.
- ↑ "Long-Term Safety and Tolerability of Acoramidis (Ag10) in Symptomatic Transthyretin Amyloid Cardiomyopathy: Updated Analysis from an Ongoing Phase 2 Open-Label Extension Study". Journal of the American College of Cardiology 79 (9): 227. March 2022. doi:10.1016/S0735-1097(22)01218-9.
- ↑ "Efficacy and Safety of Acoramidis in Transthyretin Amyloid Cardiomyopathy". The New England Journal of Medicine 390 (2): 132–142. January 2024. doi:10.1056/NEJMoa2305434. PMID 38197816. https://discovery.ucl.ac.uk/id/eprint/10185423/.
- ↑ "Program Planner". https://www.abstractsonline.com/pp8/#!/10871/presentation/16681.
- ↑ Acoramidis Achieves Early Reduction in Cardiovascular Death or Hospitalization in Transthyretin Amyloid Cardiomyopathy (ATTR-CM): Results from the ATTRibute-CM Clinical Trial OC7 (#281) (Report). 6 May 2024. doi:10.26226/m.65f9bf8ae6f73964e1d4f069.
- ↑ "BridgeBio Shares Recurrent Event Analysis of ATTRibute-CM, Demonstrating a 42% Reduction by Acoramidis on the Composite Endpoint of All-Cause Mortality and Recurrent Cardiovascular-related Hospitalization Events". https://hfsa.org/bridgebio-shares-recurrent-event-analysis-attribute-cm-demonstrating-42-reduction-acoramidis.
- ↑ "Acoramidis produces near-complete TTR stabilization in blood samples from patients with variant transthyretin amyloidosis that is greater than that achieved with tafamidis". European Heart Journal 44 (Supplement_2). November 2023. doi:10.1093/eurheartj/ehad655.989. ISSN 0195-668X.
- ↑ "Attruby (acoramidis), a Near Complete TTR Stabilizer (≥90%), approved by FDA to Reduce Cardiovascular Death and Cardiovascular-related Hospitalization in ATTR-CM Patients" (Press release). BridgeBio Pharma. 23 November 2024. Archived from the original on 25 November 2024. Retrieved 28 November 2024 – via GlobeNewswire.
- ↑ "FDA approves Stanford Medicine-developed drug that treats rare heart disease". 27 November 2024. https://med.stanford.edu/news/all-news/2024/11/spark-acoramidis.html.
- ↑ "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 83". WHO Drug Information 38 (1). 2024.
Further reading
- "AG10 inhibits amyloidogenesis and cellular toxicity of the familial amyloid cardiomyopathy-associated V122I transthyretin". Proceedings of the National Academy of Sciences of the United States of America 110 (24): 9992–9997. June 2013. doi:10.1073/pnas.1300761110. PMID 23716704. Bibcode: 2013PNAS..110.9992P.
External links
- Clinical trial number NCT03860935 for "Efficacy and Safety of AG10 in Subjects With Transthyretin Amyloid Cardiomyopathy (ATTRibute-CM)" at ClinicalTrials.gov
 |
