Chemistry:Aldoxorubicin
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C37H42N4O13 |
| Molar mass | 750.758 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
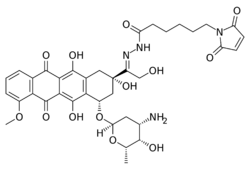
Aldoxorubicin (INNO-206) is a tumor-targeted doxorubicin conjugate in development by CytRx. Specifically, it is the (6-maleimidocaproyl) hydrazone of doxorubicin. Essentially, this chemical name describes doxorubicin attached to an acid-sensitive linker (N-ε-maleimidocaproic acid hydrazide, or EMCH).
The proposed mechanism of action is as follows:
- After administration, aldoxorubicin rapidly binds endogenous circulating albumin through the EMCH linker.
- Circulating albumin preferentially accumulates in tumors, bypassing uptake by other non-specific sites including heart, bone marrow and gastrointestinal tract.
- Once albumin-bound aldoxorubicin reaches the tumor, the acidic environment of the tumor causes cleavage of the acid sensitive linker.
- Free doxorubicin is released at the site of the tumor.
Clinical trials
Five phase I trials for safety characterization have been completed. Several phase II and III trials are underway.
Phase II
As of January 2017, there are 6 phase II clinical trials in progress:
- Second-line therapy for patients with glioblastoma[1]
- Treatment of HIV-positive patients with Kaposi's sarcoma[2]
- Combination therapy of ifosfamide and aldoxorubicin for treatment of metastatic or locally advanced sarcoma[3]
- Comparison of aldoxorubicin to the gold-standard treatment, topotecan, for metastatic small cell lung cancer[4]
- Treatment of advanced or metastatic pancreatic ductal adenocarcinoma[5]
- Comparison of aldoxorubicin and doxorubicin for patients with metastatic or locally advanced carcinoma[6]
Phase III
A phase III trial for patients with relapsed soft tissue sarcoma comparing aldoxorubicin with several other chemotherapeutics is expected to complete in 2018.[7] In November 2016, CytRx announced that preliminary results had been positive.[8]
References
- ↑ Clinical trial number NCT02014844 for "Phase 2 Study to Investigate the Efficacy and Safety of Aldoxorubicin in Subjects With Glioblastoma" at ClinicalTrials.gov
- ↑ Clinical trial number NCT02029430 for "A Study to Investigate ALDOXORUBICIN in HIV-infected Subjects With Kaposi's Sarcoma" at ClinicalTrials.gov
- ↑ Clinical trial number NCT02235701 for "Study to Investigate the Safety and Activity of Aldoxorubicin Plus Ifosfamide/Mesna in Subjects With Metastatic Soft Tissue Sarcoma" at ClinicalTrials.gov
- ↑ Clinical trial number NCT02200757 for "Efficacy and Safety of Aldoxorubicin Compared to Topotecan in Subjects With Metastatic Small Cell Lung Cancer" at ClinicalTrials.gov
- ↑ Clinical trial number NCT01580397 for "Pilot Phase 2 Study to Investigate the Preliminary Efficacy and Safety of INNO-206 in Advanced Pancreatic Cancer" at ClinicalTrials.gov
- ↑ Clinical trial number NCT01514188 for "Preliminary Efficacy and Safety of INNO-206 Compared to Doxorubicin in Advanced Soft Tissue Sarcoma" at ClinicalTrials.gov
- ↑ Clinical trial number NCT02049905 for "Phase 3 Study to Treat Patients With Soft Tissue Sarcomas" at ClinicalTrials.gov
- ↑ "CytRx (CYTR) Announces Statistically Significant Data from Aldoxorubicin Phase 3 in r/r STS". 29 November 2016. http://www.streetinsider.com/Conference+Calls/CytRx+(CYTR)+Announces+Statistically+Significant+Data+from+Aldoxorubicin+Phase+3+in+rr+STS/12289769.html.
This article includes a list of references, but its sources remain unclear because it has insufficient inline citations. (February 2018) (Learn how and when to remove this template message) |
Further reading
- "Evaluation of combination therapy schedules of doxorubicin and an acid-sensitive albumin-binding prodrug of doxorubicin in the MIA PaCa-2 pancreatic xenograft model". International Journal of Pharmaceutics 441 (1–2): 499–506. January 2013. doi:10.1016/j.ijpharm.2012.11.003. PMID 23149257.
- "Cell penetrating peptides fused to a thermally targeted biopolymer drug carrier improve the delivery and antitumor efficacy of an acid-sensitive doxorubicin derivative". International Journal of Pharmaceutics 436 (1–2): 825–32. October 2012. doi:10.1016/j.ijpharm.2012.07.043. PMID 22850291.
- "Finding the optimal balance: challenges of improving conventional cancer chemotherapy using suitable combinations with nano-sized drug delivery systems". Journal of Controlled Release 164 (2): 221–35. December 2012. doi:10.1016/j.jconrel.2012.05.045. PMID 22705248.
- "Anti-myeloma effects of the novel anthracycline derivative INNO-206". Clinical Cancer Research 18 (14): 3856–67. July 2012. doi:10.1158/1078-0432.CCR-11-3130. PMID 22619306.
 |

