Chemistry:Alkaline earth octacarbonyl complex
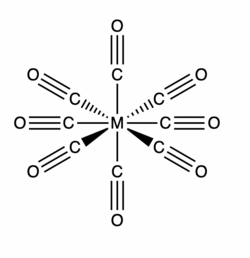
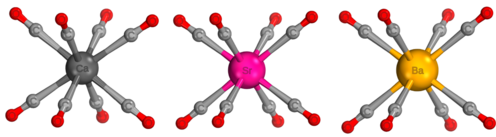
Alkaline earth octacarbonyl complexes are a class of neutral compounds that have the general formula M(CO)8 where M is a heavy Group 2 element (Ca, Sr, or Ba). The metal center has a formal oxidation state of 0 and the complex has a high level of symmetry belonging to the cubic Oh point group.[1][2] These complexes are isolable in a low-temperature neon matrix, but are not frequently used in applications due to their instability in air and water. The bonding within these complexes is controversial with some arguing the bonding resembles a model similar to bonding in transition metal carbonyl complexes which abide by the 18-electron rule,[1] and others arguing the molecule more accurately contains ionic bonds between the alkaline earth metal center and the carbonyl ligands.[3] Complexes of Be(CO)8 and Mg(CO)8 are not synthetically possible due to inaccessible (n-1)d orbitals. Beryllium has been found to form a dinuclear homoleptic carbonyl[4] and magnesium a mononuclear heteroleptic carbonyl,[5] both with only two carbonyl ligands instead of eight to each metal atom.
Synthesis and characterization
The first reported alkaline earth octacarbonyl complex, Ba(CO)8, was first synthesized by Xuan Wu and Gernot Frenking in 2018.[1] The complexes Ca(CO)8 and Sr(CO)8, were then synthesized shortly afterwards using a similar method. In the synthesis, the alkaline earth metal is produced through the ablation of a metal (Ca, Sr, or Ba) target with a laser, and then co-deposited with differing concentrations of carbon monoxide (0.02 to 0.2% in excess neon) onto a cryogenic window.[1][6] In low concentrations of carbon monoxide, low coordinate complexes such as the di-, tri- and tetracarbonyl molecules can by synthesized. Under high CO concentrations, the octacarbonyl complex is observed.
Alkaline earth carbonyl complexes are observable and characterizable through infrared spectroscopy and mass spectrometry.[7] In the infrared spectrum for the octacarbonyl complex contains only one unique carbonyl stretching band suggesting these molecules have cubic Oh symmetry.[1] Infrared spectra of octacarbonyl complexes radio-labeled with a mixture of 12C16O, 13C16O and complexes labeled with a mixture of 13C16O, and 13C18O also contain a single carbonyl stretching band indicating further successful synthesis of alkaline earth octacarbonyl complexes. In infrared spectroscopy of the complexes, the carbonyl stretching frequency is red-shifted compared to the infrared carbonyl stretch of a free CO molecule (2143 cm−1).[8] This shift could possibly be due to the strong π back-donation interaction of the metal center to the CO ligands or noncovalent intermolecular interactions between neighboring carbonyl ligands.[9][10] Transition metal carbonyl complexes also depict a red-shifted absorption peak due to π back-donation interactions. The normal range for a C≡O stretch is 1850 cm−1 to 2150 cm−1.[8] Typical mass spectra feature numerous peaks with mass to charge ratios corresponding to various [M(CO)n]+ species where n is the number of CO ligands.[1]
Structure and properties
The calculated M-CO and C≡O bond lengths from the equilibrium geometry of each M(CO)8 complex are shown in the table below.[1] Bond length between the alkaline earth center and the CO ligand increases with increasing mass of the central atom. The C≡O bond length decreases with increasing mass of the central atom. Carbonyl stretching frequencies in infrared spectroscopy occur between 1975 cm−1 to 2025 cm−1.[1] The infrared carbonyl stretch of a free CO molecule is 2143 cm−1.[8] Relative to the infrared absorption of the free CO molecule, the infrared peaks of M(CO)8 complexes are red shifted.
Alkaline earth octacarbonyl complexes are air and water sensitive.[6] They have no known applications.
| Metal Center | M-CO bond length | C≡O bond length | C≡O Stretching Frequency |
|---|---|---|---|
| Ca | 2.602 Å | 1.127 Å | 1987 cm−1 |
| Sr | 2.751 Å | 1.126 Å | 1995 cm−1 |
| Ba | 2.960 Å | 1.123 Å | 2014 cm−1 |
Bonding controversy
Group 2 alkaline earth elements have two valence electrons in an ns2 configuration, and typically use their s and p valence orbitals for bonding.[11] The heavier Group 2 elements, Ca, Sr, and Ba, in the group are capable of using their empty (n-1)d orbitals for bonding and no longer abide by the 'octet rule'. Two models for bonding in these octacarbonyl metals are possible:[1][3][10] a covalent model similar to bonding in transition metal carbonyl complexes which abide by the 18-electron rule and an ionic model where the carbonyl ligands form a salt with the alkaline earth metal. Computational methods of studying bonding interactions have produced varying results depending on the basis sets and reference states used.[3][12][13]
Covalent bonding
In this model, bonding between a CO ligand and the metal center is described using the Dewar-Chatt-Duncanson model. The CO ligand binds to the metal through σ-donation, and the metal center engages in π back-donation with the carbonyl ligand. The alkaline earth octacarbonyl complexes contain a metal center with a formal oxidation state of zero. Quantum chemical calculations using density functional theory confirm that Ca, Sr, and Ba can indeed utilize their (n-1)d in bonding to satisfy the 18-electron rule.[1][6] These computational results support the hypothesis that alkaline earth octacarbonyl complexes follow the 18-electron rule and are comparable to carbonyl transition metal complexes.
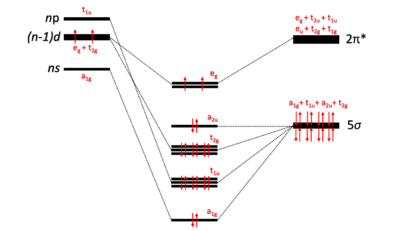
Computational methods such as QTAIM (Quantum Theory of Atoms in Molecules) and EDA-NOCV (Energy Decomposition Analysis- Natural Orbitals of Chemical Valence) analyses as well as simple electron counting support a complex that abides by the 18-electron rule. As depicted in the molecular orbital diagram above, the computed electronic structure contains a purely ligand-based orbital with a2u symmetry.[1] Invoking this ligand-only orbital allows for satisfaction of the 18-electron rule in M(CO)8 complexes, and is stabilized by the field effect of the metal on the ligand cage.[14] Alkaline earth metals are capable of adding their two valence electrons to the degenerate (n-1)d orbitals of eg symmetry.[1][6] These electrons engage in strong π back-donation with the CO ligands and account for the red-shifted CO stretching frequency in the experimentally derived infrared spectra.[15] The two electrons in the degenerate eg orbitals both have the same spin and give a triplet electronic ground state, 3A1g. This model support seven σ-donation interactions (a1g + 3t1u + 3t2g) and two π back-donation bonds (2eg) to accommodate the 18-electron rule. QTAIM provides complete sets of bond critical points and bond paths that connect the M-CO bonds in straight lines with cubic octahedral symmetry.[10] Straight lines produced by QTAIM computations are supportive evidence of covalent interactions. In M(CO)8, there are eight covalent bonding interactions between a neutral alkaline earth center and eight CO ligands produced by QTAIM.[12]
Ionic bonding
The results of quantum chemical calculations also suggest that the bonding in alkaline earth octacarbonyl complexes can be described in terms of ionic bonding between a metal center with a formal +2 oxidation state and a ligand cage with a formal charge of -2 giving the general formula: Ca2+[(CO)8]−2. In this bonding model, the carbonyl-ligand anion cage ([(CO)8]−2) serves as a σ- and π-Lewis base and the metal center acts as a Lewis acid.[3] Definitive proof of this bonding model would undermine the discovery of an alkaline earth complex that abides by the 18-electron rule.
Calculations on the bond order, bond strength, and covalent/electrostatic character of the bonds using electron localization (ELF) analysis, source function (SF) calculations, and the interacting quantum atoms (IQA) approach, concluded that M-CO bonding interactions are predominantly electrostatic in nature.[10] Covalency of the bond increases as the metal center is substituted from Ca to Sr to Ba. The bond order of all M-CO bonds were estimated to be below 1 with a low covalent contribution. In ELF calculations, there is no noticeable π back-donation contrary to the bonding interactions depicted in the Dewar-Chatt-Duncanson model.[10][13] QTAIM, RDG (Reduced Density Gradient), and DORI (Density Overlap Regions Indicator) approaches also suggest the presence of noncovalent intermolecular forces between neighboring CO groups which may give rise to the experimentally observed red-shift of the CO stretching frequencies in the infrared spectra.[11][13]
Further studies
Jellium model
In the Jellium model, the electron density and the interaction between electrons and positive charges are assumed to be evenly distributed in space. This model is used to study metal clusters. Under this model, metal clusters treated as "giant atoms", and electron energy levels interacting with the spheroid charge distribution correspond to super shells where the resulting magic numbers are 2, 8,18, 20, 32, 40.[16] The super shell configurations are depicted as capital letters (1S2, 1P6, 1D10, 2S2, 1F14, 2P6, etc.) to distinguish from the electronic shells of individual atoms.[17]
A generic octacarbonyl complex adopts cubic Oh symmetry and can be viewed as a homogenous spherical field according to the Jellium model.[14] Since full saturation of the occupied valence orbitals to form a closed shell species requires a total of 20 electrons, the magic number 20 is fulfilled. The resulting complex has the formula: [M(CO)8]q , where M is either a transition metal or alkaline earth metal and q is the charge of the ion. For all alkaline earth metals, q is -2. Since M(CO)8 complexes are not metal clusters, analogical comparison between a metal cluster previously studied under the Jellium model and M(CO)8 is required. The octa-coordinated metal cluster, [BaBe8]2− can be used with success. [BaBe8]2− has cubic Oh symmetry, contains eight coordinative bonds and two π* backdonation bonds, and contains a magic number of 20 electrons. Under the Jellium model, both complexes share similar results supporting that any theoretical ion [M(CO)8] with 20 electrons can be successfully studied under the Jellium model as a superatom and analogous to a metal cluster.[14]
In the covalent bonding model for octacarbonyl complexes, the a2u orbital is a ligand-only orbital and does not contribute to bonding (see above). Full population of the eg orbital with an additional two electrons affords the 20 electron octacarbonyl complex ([M(CO)8]−2). Results from the Jellium model support that the a2u orbital is a ligand-only orbital but contributes modestly in each coordinative M-CO bond. Transition metal octacarbonyl complexes with 20 electrons may also be studied under this model.
Impact
The synthesis of alkaline earth octacarbonyl complexes has provided insight into unconventional bonding in compounds containing alkaline earth metals that are capable of utilizing their (n-1)d orbitals. Observation of these complexes has prompted the successful exploration of other octa-coordinated alkaline earth complexes such as the octa-coordinated dinitrogen derivative: M(N2)8.[18] Computational studies are frequently used when studying bonding interactions in nonclassical molecules such as these, and development of new and improved computational methods are required to adequately resolve the bonding interaction controversy. Though computational methods have produced varying results so far, research into other complexes with unique bonding characteristics has been assisted through the study of alkaline earth octacarbonyl complexes including 225Ac-based radiopharmaceuticals and superoctahedral boranes.[19][20]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 Wu, Xuan; Zhao, Lili; Jin, Jiaye; Pan, Sudip; Li, Wei; Jin, Xiaoyang; Wang, Guanjun; Zhou, Mingfei et al. (2018-08-31). "Observation of alkaline earth complexes M(CO) 8 (M = Ca, Sr, or Ba) that mimic transition metals" (in en). Science 361 (6405): 912–916. doi:10.1126/science.aau0839. ISSN 0036-8075. PMID 30166489. Bibcode: 2018Sci...361..912W.
- ↑ Welter, Keira (August 31, 2018). "Alkaline earth carbonyls break the rules". https://www.chemistryworld.com/news/alkaline-earth-carbonyls-break-the-rules/3009449.article.
- ↑ 3.0 3.1 3.2 3.3 Landis, Clark R.; Hughes, Russell P.; Weinhold, Frank (2019-08-09). "Comment on "Observation of alkaline earth complexes M(CO) 8 (M = Ca, Sr, or Ba) that mimic transition metals"" (in en). Science 365 (6453): eaay2355. doi:10.1126/science.aay2355. ISSN 0036-8075. PMID 31395757.
- ↑ Sunil K K. "The nature of bonding and stability of beryllium carbonyl (CO)2Be-Be(CO)2: a molecule with a beryllium-beryllium double bond". J. Am. Chem. Soc. 1992, 114, 10, 3985–3986. https://doi.org/10.1021/ja00036a061
- ↑ Wang G, Gong Y, Zhang Q, Zhou M. "Formation and characterization of magnesium bisozonide and carbonyl complexes in solid argon." J Phys Chem A. 2010 Oct 14;114(40):10803-9. https://doi.org/10.1021/jp107434f. PMID 20857987.
- ↑ 6.0 6.1 6.2 6.3 6.4 Yang, Xueming (2018-11-10). "The 18-electron rule for main-group alkaline earth octacarbonyl complexes". National Science Review 6 (1): 8–9. doi:10.1093/nsr/nwy129. ISSN 2095-5138. PMID 34691820. PMC 8291552. http://dx.doi.org/10.1093/nsr/nwy129.
- ↑ "Calcium & Co. Acting Like Transition Metals :: News :: ChemistryViews" (in en). 2 September 2018. https://www.chemistryviews.org/details/news/11097361/Calcium__Co__Acting_Like_Transition_Metals.html.
- ↑ 8.0 8.1 8.2 Brown, Theodore Lawrence; Darensbourg, Donald J. (1967). "Intensities of CO stretching modes in the infrared spectra of adsorbed CO and metal carbonyls". Inorganic Chemistry 6 (5): 971–977. doi:10.1021/ic50051a026. ISSN 0020-1669. http://dx.doi.org/10.1021/ic50051a026.
- ↑ "Calcium octacarbonyl" (in en). http://chem.vander-lingen.nl/articles/Calcium_octacarbonyl/id/279/itemid/910.
- ↑ 10.0 10.1 10.2 10.3 10.4 Van der Maelen, Juan F. (2020-01-13). "Topological Analysis of the Electron Density in the Carbonyl Complexes M(CO) 8 (M = Ca, Sr, Ba)" (in en). Organometallics 39 (1): 132–141. doi:10.1021/acs.organomet.9b00699. ISSN 0276-7333. https://pubs.acs.org/doi/10.1021/acs.organomet.9b00699.
- ↑ 11.0 11.1 "Main Group Elements as Transition Metals: Alkaline Earth Octacarbonyls with 18-electrons" (in en-US). 2018-09-20. https://www.scm.com/highlights/main-group-elements-as-transition-metals-alkaline-earth-octacarbonyls-with-18-electrons/.
- ↑ 12.0 12.1 Holzmann, Nicole; Fernández, Israel; Frenking, Gernot (2020-08-24). "Comment on "Topological Analysis of the Electron Density in the Carbonyl Complexes M(CO) 8 (M = Ca, Sr, Ba)"" (in en). Organometallics 39 (16): 2956–2958. doi:10.1021/acs.organomet.0c00419. ISSN 0276-7333. https://pubs.acs.org/doi/10.1021/acs.organomet.0c00419.
- ↑ 13.0 13.1 13.2 Van der Maelen, Juan F. (2020-10-12). "Response to "Comment on 'Topological Analysis of the Electron Density in the Carbonyl Complexes M(CO) 8 (M = Ca, Sr, Ba)'"" (in en). Organometallics 39 (19): 3458–3460. doi:10.1021/acs.organomet.0c00523. ISSN 0276-7333. https://pubs.acs.org/doi/10.1021/acs.organomet.0c00523.
- ↑ 14.0 14.1 14.2 Wang, Kun; Xu, Chang; Li, Dan; Cheng, Longjiu (2020-03-26). "Applying the Jellium model to octacarbonyl metal complexes" (in en). Communications Chemistry 3 (1): 39. doi:10.1038/s42004-020-0285-2. ISSN 2399-3669. PMID 36703452.
- ↑ Koch, Daniel; Chen, Yingqian; Golub, Pavlo; Manzhos, Sergei (2019). "Revisiting π backbonding: the influence of d orbitals on metal–CO bonds and ligand red shifts". Physical Chemistry Chemical Physics 21 (37): 20814–20821. doi:10.1039/c9cp04624k. ISSN 1463-9076. PMID 31515551. Bibcode: 2019PCCP...2120814K. http://dx.doi.org/10.1039/c9cp04624k.
- ↑ Cohen, Marvin L.; Knight, Walter D. (1990-12-01). "The Physics of Metal Clusters". Physics Today 43 (12): 42–50. doi:10.1063/1.881220. ISSN 0031-9228. Bibcode: 1990PhT....43l..42C. https://physicstoday.scitation.org/doi/10.1063/1.881220.
- ↑ Economou, Eleftherios N. (2010), Economou, Eleftherios N., ed., "The Jellium Model and Metals I: Equilibrium Properties" (in en), The Physics of Solids: Essentials and Beyond, Graduate Texts in Physics (Berlin, Heidelberg: Springer): pp. 83–111, doi:10.1007/978-3-642-02069-8_4, ISBN 978-3-642-02069-8, https://doi.org/10.1007/978-3-642-02069-8_4, retrieved 2021-12-15
- ↑ Wang, Qian; Pan, Sudip; Lei, Shujun; Jin, Jiaye; Deng, Guohai; Wang, Guanjun; Zhao, Lili; Zhou, Mingfei et al. (2019-07-29). "Octa-coordinated alkaline earth metal–dinitrogen complexes M(N2)8 (M=Ca, Sr, Ba)" (in en). Nature Communications 10 (1): 3375. doi:10.1038/s41467-019-11323-5. ISSN 2041-1723. PMID 31358748. Bibcode: 2019NatCo..10.3375W.
- ↑ Fedik, Nikita; Steglenko, Dmitriy V.; Muñoz-Castro, Alvaro; Minyaev, Ruslan M.; Minkin, Vladimir I. (2021-08-12). "Band Gap Engineering and 14 Electron Superatoms in 2D Superoctahedral Boranes B4X2 (B, N, P, As, Sb)". The Journal of Physical Chemistry C 125 (31): 17280–17290. doi:10.1021/acs.jpcc.1c02939. ISSN 1932-7447. https://doi.org/10.1021/acs.jpcc.1c02939.
- ↑ Gao, Yang; Grover, Payal; Schreckenbach, Georg (2021). "Stabilization of hydrated Ac III cation: the role of superatom states in actinium-water bonding" (in en). Chemical Science 12 (7): 2655–2666. doi:10.1039/D0SC02342F. ISSN 2041-6520. PMID 34164034. PMC 8179294. http://xlink.rsc.org/?DOI=D0SC02342F.
 |