Chemistry:Ammonium phosphomolybdate
From HandWiki

| |
| Names | |
|---|---|
| Other names
Ammonium molybdophosphate
Triammonium 12-molybdophosphate | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| (NH4)3PMo12O40 | |
| Molar mass | 1876.35 g/mol |
| Appearance | Yellow crystals |
| Melting point | Decomposes |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335[2] | |
| P261, P305+351+338[2] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
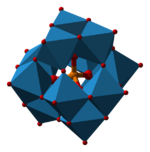
Ammonium phosphomolybdate is the inorganic salt of phosphomolybdic acid with the chemical formula (NH4)3PMo12O40. The salt contains the phosphomolybdate anion, a well known heteropolymetalate of the Keggin structural class.
Synthesis
Ammonium phosphomolybdate can be made by heating ammonium orthomolybdate combined with phosphoric acid and nitric acid, yielding ammonium nitrate, water, and a yellow precipitate of ammonium phosphomolybdate is obtained.[3]
- 12 (NH
4)
6Mo
7O
24(H
2O)
4 + 7 Na
2HPO
4(H
2O) + 65 HNO
3 → 7 (NH
4)
3Mo
12PO
40 + 51 NH
4NO
3 + 14 NaNO
3 + 56 H
2O
Normally, it often is as a hexahydrate, which is a dark yellow fine crystal, and poorly soluble in water.[3]
References
- ↑ ChemicalBook: http://www.chemicalbook.com/ChemicalProductProperty_EN_CB6162083.htm
- ↑ 2.0 2.1 "Ammonium phosphomolybdate hydrate". http://www.sigmaaldrich.com/catalog/product/aldrich/342165.
- ↑ 3.0 3.1 Dias, José Alves; Dias, Sílvia Cláudia Loureiro; Caliman, Ednéia (2014). "Keggin Structure Polyoxometalates". Inorganic Syntheses: Volume 36. 36. pp. 210–217. doi:10.1002/9781118744994.ch39. ISBN 9781118744994.
 |