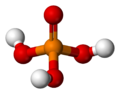
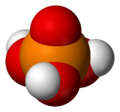
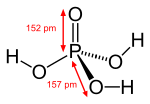
Chemistry:Phosphoric acid
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Phosphoric acid
| |||
| Other names
Orthophosphoric acid, hydrogen phosphate
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| EC Number |
| ||
| 2000 | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1805 | ||
| |||
| |||
| Properties | |||
| H 3PO 4 | |||
| Molar mass | 97.994 g·mol−1 | ||
| Appearance | Colorless solid | ||
| Odor | Odorless | ||
| Density | 1.6845 g/cm3 (25 °C, 85%),[1] 1.834 g/cm3 (solid)[2] | ||
| Melting point | 42.35 °C (108.23 °F; 315.50 K) anhydrous[12] 29.32 °C (84.78 °F; 302.47 K) hemihydrate[13] | ||
| Boiling point | |||
| Solubility | Soluble in ethanol | ||
| log P | −2.15[7] | ||
| Vapor pressure | 0.03 mmHg (20 °C)[8] | ||
| Conjugate base | Dihydrogen phosphate | ||
| −43.8·10−6 cm3/mol[10] | |||
Refractive index (nD)
|
| ||
| Viscosity | 2.4–9.4 cP (85% aq. soln.) 147 cP (100%) | ||
| Structure | |||
| Monoclinic | |||
| Tetrahedral | |||
| Thermochemistry[14] | |||
Heat capacity (C)
|
145.0 J/(mol⋅K) | ||
Std molar
entropy (S |
150.8 J/(mol⋅K) | ||
Std enthalpy of
formation (ΔfH⦵298) |
−1271.7 kJ/mol | ||
Gibbs free energy (ΔfG˚)
|
−1123.6 kJ/mol | ||
| Hazards | |||
| Safety data sheet | ICSC 1008 | ||
| GHS pictograms |  [15] [15]
| ||
| GHS Signal word | Danger | ||
| H290, H314[15] | |||
| P280, P305+351+338, P310[15] | |||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
1530 mg/kg (rat, oral)[16] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 1 mg/m3[8] | ||
REL (Recommended)
|
TWA 1 mg/m3 ST 3 mg/m3[8] | ||
IDLH (Immediate danger)
|
1000 mg/m3[8] | ||
| Related compounds | |||
Related phosphorus oxoacids
|
| ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula H
3PO
4. It is commonly encountered as an 85% aqueous solution, which is a colourless, odourless, and non-volatile syrupy liquid. It is a major industrial chemical, being a component of many fertilizers.
The compound is an acid. Removal of all three H+
ions gives the phosphate ion PO3−
4. Removal of one or two protons gives dihydrogen phosphate ion H
2PO−
4, and the hydrogen phosphate ion HPO2−
4, respectively. Phosphoric acid forms esters, called organophosphates.[17]
The name "orthophosphoric acid" can be used to distinguish this specific acid from other "phosphoric acids", such as pyrophosphoric acid. Nevertheless, the term "phosphoric acid" often means this specific compound; and that is the current IUPAC nomenclature.
Production
Phosphoric acid is produced industrially by one of two routes, wet processes and dry.[18][19][20]
Wet process
In the wet process, phosphate-containing minerals such as calcium hydroxyapatite or fluorapatite are treated with sulfuric acid.[21]
- Ca
5(PO
4)
3OH + 5 H
2SO
4 → 3 H
3PO
4 + 5 CaSO
4 + H
2O - Ca
5(PO
4)
3F + 5 H
2SO
4 → 3 H
3PO
4 + 5 CaSO
4 + HF
By-products include calcium sulfate (CaSO
4) and hydrogen fluoride (HF). The HF gas may be recovered by streaming it into a wet (water) scrubber producing hydrofluoric acid. CaSO
4 is better known as gypsum, which is commonly used in the construction industry, however the CaSO
4 produced from phosphoric acid production can contain trace levels of radioactive elements such as radium. This makes it unsuitable for commercial use, and it is called phosphogypsum to distinguish it. It is typically stored indefinitely.
In both cases the phosphoric acid solution usually contains 23–33% P
2O
5 (32–46% H
3PO
4). It may be concentrated to produce commercial- or merchant-grade phosphoric acid, which contains about 54–62% P
2O
5 (75–85% H
3PO
4). Further removal of water yields superphosphoric acid with a P
2O
5 concentration above 70% (corresponding to nearly 100% H
3PO
4). The phosphoric acid from both processes may be further purified by removing compounds of arsenic and other potentially toxic impurities.
Dry process
To produce food-grade phosphoric acid, phosphate ore is first reduced with coke in an electric arc furnace, to give elemental phosphorus. This process is also known as the thermal process or the electric furnace process. Silica is also added, resulting in the production of calcium silicate slag. Elemental phosphorus is distilled out of the furnace and burned with air to produce high-purity phosphorus pentoxide, which is dissolved in water to make phosphoric acid.[22] The thermal process produces phosphoric acid with a very high concentration of P
2O
5 (about 85%) and a low level of impurities.
However, this process is more expensive and energy-intensive than the wet process, which produces phosphoric acid with a lower concentration of P
2O
5 (about 26–52%) and a higher level of impurities. The wet process is the most common method of producing phosphoric acid for fertilizer use.[23] Even in China, where the thermal process is still used quite widely due to relatively cheap coal as opposed to the sulfuric acid, over 7/8 of phosphoric acid is produced with wet process.[24]
Purification
Phosphoric acid produced from phosphate rock or thermal processes often requires purification. A common purification method is liquid–liquid extraction, which involves the separation of phosphoric acid from water and other impurities using organic solvents, such as tributyl phosphate (TBP), methyl isobutyl ketone (MIBK), or n-octanol. Nanofiltration involves the use of a premodified nanofiltration membrane, which is functionalized by a deposit of a high molecular weight polycationic polymer of polyethyleneimines. Nanofiltration has been shown to significantly reduce the concentrations of various impurities, including cadmium, aluminum, iron, and rare earth elements. The laboratory and industrial pilot scale results showed that this process allows the production of food-grade phosphoric acid.[25]
Fractional crystallization can achieve higher purities typically used for semiconductor applications. Usually a static crystallizer is used. A static crystallizer uses vertical plates, which are suspended in the molten feed and which are alternatingly cooled and heated by a heat transfer medium. The process begins with the slow cooling of the heat transfer medium below the freezing point of the stagnant melt. This cooling causes a layer of crystals to grow on the plates. Impurities are rejected from the growing crystals and are concentrated in the remaining melt. After the desired fraction has been crystallized, the remaining melt is drained from the crystallizer. The purer crystalline layer remains adhered to the plates. In a subsequent step, the plates are heated again to liquify the crystals and the purified phosphoric acid drained into the product vessel. The crystallizer is filled with feed again and the next cooling cycle is started.[26]
Properties
Acidic properties
In aqueous solution phosphoric acid behaves as a triprotic acid.
- H
3PO
4 ⇌ H
2PO−
4 + H+
, pKa1 = 2.14 - H
2PO−
4 ⇌ HPO2−
4 + H+
, pKa2 = 7.20 - HPO2−
4 ⇌ PO3−
4 + H+
, pKa3 = 12.37
The difference between successive pKa values is sufficiently large so that salts of either monohydrogen phosphate, HPO2−
4 or dihydrogen phosphate, H
2PO−
4, can be prepared from a solution of phosphoric acid by adjusting the pH to be mid-way between the respective pKa values.
Aqueous solutions
Aqueous solutions up to 62.5% H
3PO
4 are eutectic, exhibiting freezing-point depression as low as −85 °C. When the concentration of acid rises above 62.5% the freezing-point increases, reaching 21 °C by 85% H
3PO
4 (w/w; the monohydrate). Beyond this the phase diagram becomes complicated, with significant local maxima and minima. For this reason phosphoric acid is rarely sold above 85%, as beyond this adding or removing small amounts of moisture risks the entire mass freezing solid, which would be a major problem on a large scale. A local maximum at 91.6% which corresponds to the hemihydrate 2H3PO4•H2O, freezing at 29.32 °C.[27][28] There is a second smaller eutectic depression at a concentration of 94.75% with a freezing point of 23.5 °C. At higher concentrations the freezing point rapidly increases. Concentrated phosphoric acid tends to supercool before crystallization occurs, and may be relatively resistant to crystallisation even when stored below the freezing point.[13]
Self condensation
Phosphoric acid is commercially available as aqueous solutions of various concentrations, not usually exceeding 85%. If concentrated further it undergoes slow self-condensation, forming an equilibrium with pyrophosphoric acid:
- 2 H
3PO
4 ⇌ H
2O + H
4P
2O
7
Even at 90% concentration the amount of pyrophosphoric acid present is negligible, but beyond 95% it starts to increase, reaching 15% at what would have otherwise been 100% orthophosphoric acid.[29]
As the concentration is increased higher acids are formed, culminating in the formation of polyphosphoric acids.[30] It is not possible to fully dehydrate phosphoric acid to phosphorus pentoxide, instead the polyphosphoric acid becomes increasingly polymeric and viscous. Due to the self-condensation, pure orthophosphoric acid can only be obtained by a careful fractional freezing/melting process.[13][12]
Uses
The dominant use of phosphoric acid is for fertilizers, consuming approximately 90% of production.[31]
| Application | Demand (2006) in thousands of tons | Main phosphate derivatives |
|---|---|---|
| Soaps and detergents | 1836 | STPP |
| Food industry | 309 | STPP (Na 5P 3O 10), SHMP, TSP, SAPP, SAlP, MCP, DSP (Na 2HPO 4), H 3PO 4 |
| Water treatment | 164 | SHMP, STPP, TSPP, MSP (NaH 2PO 4), DSP |
| Toothpastes | 68 | DCP (CaHPO 4), IMP, SMFP |
| Other applications | 287 | STPP (Na 3P 3O 9), TCP, APP, DAP, zinc phosphate (Zn 3(PO 4) 2), aluminium phosphate (AlPO 4), H 3PO 4 |
Food-grade phosphoric acid (additive E338[32]) is used to acidify foods and beverages such as various colas and jams, providing a tangy or sour taste. The phosphoric acid also serves as a preservative.[33] Soft drinks containing phosphoric acid, which would include Coca-Cola, are sometimes called phosphate sodas or phosphates. Phosphoric acid in soft drinks has the potential to cause dental erosion.[34] Phosphoric acid also has the potential to contribute to the formation of kidney stones, especially in those who have had kidney stones previously.[35]
Specific applications of phosphoric acid include:
- in anti-rust treatment by phosphate conversion coating or passivation
- to prevent iron oxidation by means of the Parkerization process
- as an external standard for phosphorus-31 nuclear magnetic resonance
- in phosphoric acid fuel cells
- in activated carbon production[36]
- in compound semiconductor processing, to etch Indium gallium arsenide selectively with respect to indium phosphide[37]
- in microfabrication to etch silicon nitride selectively with respect to silicon dioxide[38]
- in microfabrication to etch aluminium
- as a pH adjuster in cosmetics and skin-care products[39]
- as a sanitizing agent in the dairy, food, and brewing industries[40]
Phosphoric acid may also be used for chemical polishing (etching) of metals like aluminium or for passivation of steel products in a process called phosphatization.[41]
Safety
Phosphoric acid is not a strong acid. However, at moderate concentrations phosphoric acid solutions are irritating to the skin. Contact with concentrated solutions can cause severe skin burns and permanent eye damage.[42]
A link has been shown between long-term regular cola intake and osteoporosis in later middle age in women (but not men).[43]
See also
- Phosphate fertilizers, such as ammonium phosphate fertilizers
- Chiral phosphoric acid
References
- ↑ Christensen, J. H.; Reed, R. B. (1955). "Design and Analysis Data—Density of Aqueous Solutions of Phosphoric Acid Measurements at 25 °C.". Ind. Eng. Chem. 47 (6): 1277–1280. doi:10.1021/ie50546a061.
- ↑ "CAMEO Chemicals Datasheet – Phosphoric Acid". https://cameochemicals.noaa.gov/chemical/4231.
- ↑ "Phosphoric acid". http://www.chemspider.com/Chemical-Structure.979.html.
- ↑ Brown, Earl H.; Whitt, Carlton D. (1952). "Vapor Pressure of Phosphoric Acids". Industrial & Engineering Chemistry 44 (3): 615–618. doi:10.1021/ie50507a050. https://pubs.acs.org/doi/pdf/10.1021/ie50507a050.
- ↑ Seidell, Atherton; Linke, William F. (1952). Solubilities of Inorganic and Organic Compounds. Van Nostrand. https://books.google.com/books?id=k2e5AAAAIAAJ. Retrieved 2 June 2014.
- ↑ Haynes, p. 4.80
- ↑ "phosphoric acid_msds". https://www.chemsrc.com/en/cas/7664-38-2_329226.html.
- ↑ 8.0 8.1 8.2 8.3 NIOSH Pocket Guide to Chemical Hazards. "#0506". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0506.html.
- ↑ Haynes, p. 5.92
- ↑ Haynes, p. 4.134
- ↑ Edwards, O. W.; Dunn, R. L.; Hatfield, J. D. (1964). "Refractive Index of Phosphoric Acid Solutions at 25 C.". J. Chem. Eng. Data 9 (4): 508–509. doi:10.1021/je60023a010.
- ↑ 12.0 12.1 Greenwood, N. N.; Thompson, A. (1959). "701. The mechanism of electrical conduction in fused phosphoric and trideuterophosphoric acids". Journal of the Chemical Society (Resumed): 3485. doi:10.1039/JR9590003485.
- ↑ 13.0 13.1 13.2 Ross, Wm. H.; Jones, R. M.; Durgin, C. B. (October 1925). "The Purification of Phosphoric Acid by Crystallization." (in en). Industrial & Engineering Chemistry 17 (10): 1081–1083. doi:10.1021/ie50190a031. ISSN 0019-7866. https://pubs.acs.org/doi/abs/10.1021/ie50190a031.
- ↑ Haynes, p. 5.13
- ↑ 15.0 15.1 15.2 Sigma-Aldrich Co., Phosphoric acid.
- ↑ "Phosphoric acid". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/7664382.html.
- ↑ Westheimer, F.H. (6 June 1987). "Why nature chose phosphates". Science 235 (4793): 1173–1178 (see pp. 1175–1176). doi:10.1126/science.2434996. PMID 2434996. Bibcode: 1987Sci...235.1173W.
- ↑ Becker, Pierre (1988). Phosphates and phosphoric acid. New York: Marcel Dekker. ISBN 978-0824717124.
- ↑ Gilmour, Rodney (2014). Phosphoric acid: purification, uses, technology, and economics. Boca Raton: CRC Press. pp. 44–61. ISBN 9781439895108. https://books.google.com/books?id=lDMTAgAAQBAJ&pg=PP1.
- ↑ Jupp, Andrew R.; Beijer, Steven; Narain, Ganesha C.; Schipper, Willem; Slootweg, J. Chris (2021). "Phosphorus recovery and recycling – closing the loop". Chemical Society Reviews 50 (1): 87–101. doi:10.1039/D0CS01150A. PMID 33210686.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 520–522. ISBN 978-0-08-037941-8.
- ↑ Geeson, Michael B.; Cummins, Christopher C. (2020). "Let's Make White Phosphorus Obsolete". ACS Central Science 6 (6): 848–860. doi:10.1021/acscentsci.0c00332. PMID 32607432.
- ↑ Phosphoric Acid and Phosphatic Fertilizers: A profile
- ↑ Minpeng; Chen; Fu; Sun; Xu; Xia; Ji-ning. "The Phosphorus Flow in China : A Revisit from the Perspective of Production". Global Environmental Research 19 (1): 19–25. https://airies.wikiplus.net/attach.php/6a6f75726e616c5f31392d31656e67/save/0/0/19_1-4.pdf.
- ↑ Wet Process Phosphoric Acid Purification (2022). "Wet Process Phosphoric Acid Purification Using Functionalized Organic Nanofiltration Membrane". Separations 9 (4): 100. doi:10.3390/separations9040100.
- ↑ Fractional Crystallization
- ↑ Ross, William H.; Jones, Russell M. (August 1925). "The Solubility and Freezing-Point Curves of Hydrated and Anhydrous Orthophosphoric Acid". Journal of the American Chemical Society 47 (8): 2165–2170. doi:10.1021/ja01685a015. Bibcode: 1925JAChS..47.2165R.
- ↑ "Purified Phosphoric Acid H3PO4 Technical Information Bulletin". PotashCorp. http://www.waterguardinc.com/files/90712047.pdf.
- ↑ Korte, Carsten; Conti, Fosca; Wackerl, Jürgen; Lehnert, Werner (2016), Li, Qingfeng; Aili, David; Hjuler, Hans Aage et al., eds., "Phosphoric Acid and its Interactions with Polybenzimidazole-Type Polymers" (in en), High Temperature Polymer Electrolyte Membrane Fuel Cells (Cham: Springer International Publishing): pp. 169–194, doi:10.1007/978-3-319-17082-4_8, ISBN 978-3-319-17081-7, http://link.springer.com/10.1007/978-3-319-17082-4_8, retrieved 2023-02-12
- ↑ Jameson, R. F. (1 January 1959). "151. The composition of the "strong" phosphoric acids". Journal of the Chemical Society (Resumed): 752–759. doi:10.1039/JR9590000752.
- ↑ Schrödter, Klaus; Bettermann, Gerhard; Staffel, Thomas; Wahl, Friedrich; Klein, Thomas; Hofmann, Thomas (2008). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_465.pub3.
- ↑ "Current EU approved additives and their E Numbers". Foods Standards Agency. 14 March 2012. http://www.food.gov.uk/policy-advice/additivesbranch/enumberlist#h_7.
- ↑ "Why is phosphoric acid used in some Coca‑Cola drinks?| Frequently Asked Questions | Coca-Cola GB" (in en-GB). https://www.coca-cola.co.uk/our-business/faqs/why-is-phosphoric-acid-used-in-coca-cola-drinks-diet-coke-coke-zero.
- ↑ Moynihan, P. J. (23 November 2002). "Dietary advice in dental practice". British Dental Journal 193 (10): 563–568. doi:10.1038/sj.bdj.4801628. PMID 12481178.
- ↑ Qaseem, A; Dallas, P; Forciea, MA; Starkey, M et al. (4 November 2014). "Dietary and pharmacologic management to prevent recurrent nephrolithiasis in adults: A clinical practice guideline from the American College of Physicians". Annals of Internal Medicine 161 (9): 659–67. doi:10.7326/M13-2908. PMID 25364887.
- ↑ Toles, C.; Rimmer, S.; Hower, J. C. (1996). "Production of activated carbons from a washington lignite using phosphoric acid activation". Carbon 34 (11): 1419. doi:10.1016/S0008-6223(96)00093-0. Bibcode: 1996Carbo..34.1419T.
- ↑ Wet chemical etching. umd.edu.
- ↑ Wolf, S.; R. N. Tauber (1986). Silicon processing for the VLSI era: Volume 1 – Process technology. Lattice Press. p. 534. ISBN 978-0-9616721-6-4.
- ↑ "Ingredient dictionary: P". Cosmetic ingredient dictionary. Paula's Choice. http://www.cosmeticscop.com/learn/cosmetic_dictionary.asp?id=21&letter=P.
- ↑ "Star San". Five Star Chemicals. http://www.fivestarchemicals.com/wp-content/uploads/StarSanTech-HB2.pdf.
- ↑ "Phosphates - Metal Finishing". Phospates for Americas. February 2021. https://phosphatesfacts.org/wp-content/uploads/2021/02/Phosphates-Metal-Finishing.pdf.
- ↑ "Phosphoric Acid, 85 wt.% SDS". 5 May 2016. http://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=US&language=en&productNumber=345245&brand=ALDRICH&PageToGoToURL=http%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fproduct%2Faldrich%2F345245%3Flang%3Den.
- ↑ "Colas, but not other carbonated beverages, are associated with low bone mineral density in older women: The Framingham Osteoporosis Study". American Journal of Clinical Nutrition 84 (4): 936–942. 1 October 2006. doi:10.1093/ajcn/84.4.936. PMID 17023723.
Cited sources
- Haynes, William M., ed (2011). CRC Handbook of Chemistry and Physics (92nd ed.). CRC Press. ISBN 978-1439855119.
External links
 |