Chemistry:Andrographolide
Andrographolide is a labdane diterpenoid that has been isolated from the stem and leaves of Andrographis paniculata.[1] Andrographolide is an extremely bitter substance.
Andrographolide has been studied for its effects on cell signaling, immunomodulation, and stroke.[2] Study has shown that andrographolide may bind to a spectrum of protein targets including NF-κB and actin by covalent modification.[3][4]
Andrographolide is a non-ATP-competitive, substrate-competitive GSK-3β inhibitor.[5][6][7] When the substrate concentration is 25μM, the IC50 of andrographolide for GSK-3β is 5.58±0.40μM. When the substrate concentration is increased to 90μM, the IC50 increases to 37.7μM.[5]
Biosynthesis
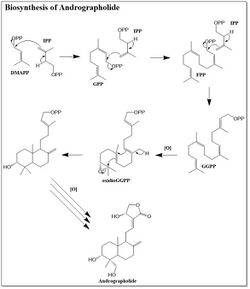
While andrographolide is a relatively simple diterpene lactone, the biosynthesis by Andrographis paniculata was determined in the 2010s.[8][9] Andrographolide is a member of the isoprenoid family of natural products. The precursors to isoprenoid biosynthesis, isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), can be synthesized through either the mevalonic acid pathway (MVA) or deoxyxylulose pathway (DXP).[10] Through selective C13 labeling of the precursors to both the MVA and DXP pathways, it was determined that the majority of the andrographolide precursors are synthesized through the DXP pathway.[10] There are a small portion of andrographolide precursors synthesized through the MVA pathway. The biosynthesis of andrographolide begins with the addition of IPP to DMAPP, which forms geranyl pyrophosphate. Another molecule of IPP is then added, yielding farnesyl pyrophosphate (FPP). The final IPP molecule is added to the FPP to complete the backbone of the diterpene. The double bond originating from DMAPP is oxidized to an epoxide prior to the ring closing cascade that forms two six-membered rings. A series of oxidations form a five-membered lactone in addition to adding on the alcohol groups. The order of these post-synthetic modifications is not entirely known.[10]
Research
In 2022, a research found that the combinatorial drug of guaifenesin and andrographolide has potential anticonvulsant activity using network virtual screening. The possible mechanism of action is the two drugs synergistically affect NMDA receptors.[11]
See also
- Xiyanping WINDOSE-Tablet
References
- ↑ "Andrographolide, the active constituent of Andrographis paniculata Nees; a preliminary communication". The Indian Medical Gazette 86 (3): 96–7. March 1951. PMID 14860885.
- ↑ abcamBiochemicals Andrographolide-ab120636
- ↑ "A quantitative chemical proteomics approach to profile the specific cellular targets of andrographolide, a promising anticancer agent that suppresses tumor metastasis". Molecular & Cellular Proteomics 13 (3): 876–86. March 2014. doi:10.1074/mcp.M113.029793. PMID 24445406.
- ↑ "Is there a future for andrographolide to be an anti-inflammatory drug? Deciphering its major mechanisms of action". Biochemical Pharmacology 139: 71–81. September 2017. doi:10.1016/j.bcp.2017.03.024. PMID 28377280.
- ↑ 5.0 5.1 Cheril Tapia-Rojas, Andreas Schüller, Carolina B. Lindsay, Roxana C. Ureta, Cristóbal Mejías-Reyes, Juan Hancke, Francisco Melo, Nibaldo C. Inestrosa (1 March 2015). "Andrographolide activates the canonical Wnt signalling pathway by a mechanism that implicates the non-ATP competitive inhibition of GSK-3β: autoregulation of GSK-3β in vivo". Biochem J 466 (2): 415–430. doi:10.1042/BJ20140207. https://www.professionalnutritionals.com/wp-content/uploads/2015/11/Andrographolide-Activates-the-Canonical-Wnt-Pathway.pdf.
- ↑ Zheng Zhao, Ye Yuan, Shuang Li, Xiaofeng Wang, Xue Yang (11 August 2024). "Natural compounds from herbs and nutraceuticals as glycogen synthase kinase-3β inhibitors in Alzheimer's disease treatment". CNS Neuroscience & Therapeutics 30 (8). doi:10.1111/cns.14885. PMC 11317746. https://onlinelibrary.wiley.com/doi/full/10.1111/cns.14885.
- ↑ Hassan WRM, Ali AH, Basir R, Embi N, Sidek HM (1 September 2022). "Medicinal plants with antimalarial activities mediated via glycogen synthase kinase-3 beta (GSK3β) inhibition". Trop Biomed 39 (3): 384-393. doi:10.47665/tb.39.3.008. PMID 36214435. https://msptm.org/files/Vol39No3/tb-39-3-008-Hassan-W-R-M.pdf.
- ↑ "Andrographis paniculata transcriptome provides molecular insights into tissue-specific accumulation of medicinal diterpenes". BMC Genomics 16 (1): 659. September 2015. doi:10.1186/s12864-015-1864-y. PMID 26328761.
- ↑ "Jasmonate-induced biosynthesis of andrographolide in Andrographis paniculata". Physiologia Plantarum 153 (2): 221–9. February 2015. doi:10.1111/ppl.12252. PMID 25104168.
- ↑ 10.0 10.1 10.2 "Biosynthesis of andrographolide in Andrographis paniculata". Phytochemistry 71 (11–12): 1298–304. August 2010. doi:10.1016/j.phytochem.2010.05.022. PMID 20557910. Bibcode: 2010PChem..71.1298S.
- ↑ Yunyuan Huang, Haiyan Xu, Pei Wang, Rurong Gu, Xiaokang Li, Yixiang Xu, Jiqun Wang, Sicong Qiao, Donglei Shi, Zhaobing Gao, Jian Li (2022-03-25). "Identification of Guaifenesin–Andrographolide as a Novel Combinatorial Drug Therapy for Epilepsy Using Network Virtual Screening and Experimental Validation". ACS Chemical Neuroscience 13 (7). doi:10.1021/acschemneuro.1c00774. https://pubs.acs.org/doi/10.1021/acschemneuro.1c00774.
 |
