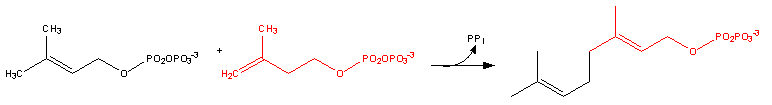Chemistry:Farnesyl pyrophosphate
| |
| Names | |
|---|---|
| Preferred IUPAC name
(2E,6E)-3,7,11-Trimethyldodeca-2,6,10-trien-1-yl trihydrogen diphosphate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| MeSH | farnesyl+pyrophosphate |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C15H28O7P2 | |
| Molar mass | 382.330 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Farnesyl pyrophosphate (FPP), also known as farnesyl diphosphate (FDP), is an intermediate in the biosynthesis of terpenes and terpenoids such as sterols and carotenoids.[1] It is also used in the synthesis of CoQ (part of the electron transport chain), as well as dehydrodolichol diphosphate (a precursor of dolichol, which transports proteins to the ER lumen for N-glycosylation).
Biosynthesis
Farnesyl pyrophosphate synthase (a prenyl transferase)[2] catalyzes sequential condensation reactions of dimethylallyl pyrophosphate with 2 units of 3-isopentenyl pyrophosphate to form farnesyl pyrophosphate, as is shown in the following two steps:
- Dimethylallyl pyrophosphate reacts with 3-isopentenyl pyrophosphate to form geranyl pyrophosphate:
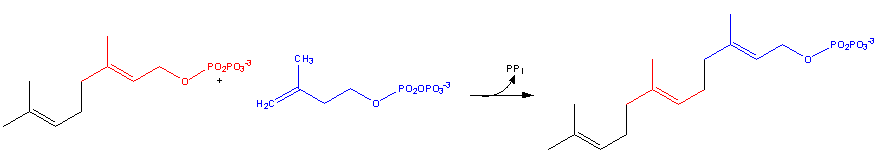
- Geranyl pyrophosphate then reacts with another molecule of 3-isopentenyl pyrophosphate to form farnesyl pyrophosphate
Pharmacology
The above reactions are inhibited by bisphosphonates (used for osteoporosis).[3] Farnesyl pyrophosphate is a selective agonist of TRPV3.[4]
Related compounds
References
- ↑ "Cyclization enzymes in the biosynthesis of monoterpenes, sesquiterpenes, and diterpenes". Topics in Current Chemistry 209: 53–95. 2000. doi:10.1007/3-540-48146-X_2. ISBN 978-3-540-66573-1.
- ↑ "Characterization of three novel isoprenyl diphosphate synthases from the terpenoid rich mango fruit". Plant Physiology and Biochemistry 71: 121–131. October 2013. doi:10.1016/j.plaphy.2013.07.006. PMID 23911730.
- ↑ "Bisphosphonates: from bench to bedside". Annals of the New York Academy of Sciences 1068 (April 2006): 367–401. April 2006. doi:10.1196/annals.1346.041. PMID 16831938. Bibcode: 2006NYASA1068..367R.
- ↑ "Farnesyl pyrophosphate is a novel pain-producing molecule via specific activation of TRPV3". The Journal of Biological Chemistry 285 (25): 19362–71. June 2010. doi:10.1074/jbc.M109.087742. PMID 20395302.
 |