Chemistry:Arsenicin A
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2,4,6-Trioxa-1,3,5,7-tetraarsaadamantane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C3H6As4O3 | |||
| Molar mass | 389.764 g·mol−1 | ||
| Melting point | 182 to 184 °C (360 to 363 °F; 455 to 457 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
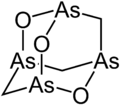
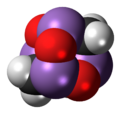
Arsenicin A is a naturally occurring arsenic heterocycle with the molecular formula C3H6As4O3. It was first isolated from the New Caledonian marine sponge Echinochalina bargibanti.[1] The compound was characterized by computational and spectroscopic[2][3] techniques and found to possess a cage-like structure similar to adamantane in which the four methanetriyl carbon bridgeheads are replaced by arsenic atoms and three of the six methylene bridges are replaced by oxygen atoms. It is the first polyarsenic compound ever found in nature.[1] Subsequently, the proposed structure was prepared in large quantities via total synthesis and the structure was confirmed by x-ray crystallography.[4][5] The molecule is chiral, and has been resolved into its two enantiomers.[5] Arsenicin A is active against promelocytic leukemia cells at lower concentrations than the arsenic(III) oxide drug Trisenox.[5]
References
- ↑ 1.0 1.1 Mancini, Ines; Guella, Graziano; Frostin, Maryvonne; Hnawia, Edouard; Laurent, Dominique; Debitus, Cecile; Pietra, Francesco (2006). "On the First Polyarsenic Organic Compound from Nature: Arsenicin a from the New Caledonian Marine Sponge Echinochalina bargibanti". Chemistry: A European Journal 12 (35): 8989–94. doi:10.1002/chem.200600783. PMID 17039560.
- ↑ Tähtinen, Petri; Saielli, Giacomo; Guella, Graziano; Mancini, Ines; Bagno, Alessandro (2008). "Computational NMR Spectroscopy of Organoarsenicals and the Natural Polyarsenic Compound Arsenicin A". Chemistry: A European Journal 14 (33): 10445–52. doi:10.1002/chem.200801272. PMID 18846604.
- ↑ Guella, Graziano; Mancini, Ines; Mariotto, Gino; Rossi, Barbara; Viliani, Gabriele (2009). "Vibrational analysis as a powerful tool in structure elucidation of polyarsenicals: a DFT-based investigation of arsenicin A". Physical Chemistry Chemical Physics 11 (14): 2420–2427. doi:10.1039/b816729j. PMID 19325974. Bibcode: 2009PCCP...11.2420G.
- ↑ Di Lu; A. David Rae; Geoff Salem; Michelle L. Weir; Anthony C. Willis; S. Bruce Wild (2010). "Arsenicin A, A Natural Polyarsenical: Synthesis and Crystal Structure". Organometallics 29 (1): 32–33. doi:10.1021/om900998q.
- ↑ 5.0 5.1 5.2 Lu, Di; Coote, Michelle L.; Ho, Junming; Kilah, Nathan L.; Lin, Ching-Yeh; Salem, Geoff; Weir, Michelle L.; Willis, Anthony C. et al. (2012-03-12). "Resolution and Improved Synthesis of (±)-Arsenicin A: A Natural Adamantane-Type Tetraarsenical Possessing Strong Anti-Acute Promelocytic Leukemia Cell Line Activity". Organometallics 31 (5): 1808–1816. doi:10.1021/om201180d.
 |