Chemistry:Azaborane
From HandWiki
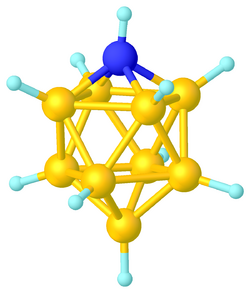
9H
10[1]
Azaborane usually refers a borane cluster where BH vertices are replaced by N or NR (R stands typically for H or organic substituent). Like many of the related boranes, these clusters are polyhedra and can be classified as closo-, nido-, arachno-, etc.
Within the context of Wade's rules, NR is a 4-electron vertex, and N is a 3-electron vertex. Prominent examples are the charge-neutral nido-NB
10H
13 (i.e. (NH)(BH)
10) and closo-NB
11H
12 (i.e. (NH)(BH)
11).[2]
Azaboranes can also refer to simpler compounds including iminoboranes (RB=NR', where R and R' stand typically for H or organic substituent) and borazines.
See also
References
- ↑ Lenka Schneider; Ulli Englert; Peter Paetzold (1994). "Die Kristallstruktur von Aza‐closo‐decaboran NB9H10". Z. Anorg. Allg. Chem. 620 (7): 1191–1193. doi:10.1002/zaac.19946200711.
- ↑ P. Paetzold (1991). "New Perspectives in Boron-Nitrogen Chemistry-I". Pure Appl. Chem. 63 (3): 345–350. doi:10.1351/pac199163030345. https://www.iupac.org/publications/pac/pdf/1991/pdf/6303x0345.pdf.
 |
