Chemistry:Azomethane
From HandWiki
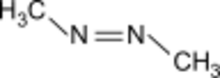
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C2H6N2 | |
| Molar mass | 58.084 g·mol−1 |
| Appearance | colourless to pale yellow gas[1] |
| Melting point | −78 °C (trans)[2] −66 °C (cis)[2] |
| Boiling point | 1.5 °C (trans)[2] 95 °C (cis)[2] |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H220, H280 | |
| P203Script error: No such module "Preview warning".Category:GHS errors, P210, P222, P280, P377, P381, P403, P410+403 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Azomethane is an organic compound with the chemical formula CH3-N=N-CH3. It exhibits cis-trans isomerism. It can be produced by the reaction of 1,2-dimethylhydrazine dihydrochloride with copper(II) chloride in sodium acetate solution. The reaction produces the azomethane complex of copper(I) chloride, which can produce free azomethane by thermal decomposition.[4] It is the source of methyl radical in laboratory.[5]
- CH3-N=N-CH3 → 2 CH3· + N2
References
- ↑ Azomethan Lexikon der Chemie
- ↑ 2.0 2.1 2.2 2.3 Ackermann, Martin N.; Craig, Norman C.; Isberg, Ralph R.; Lauter, David M.; MacPhail, Richard A.; Young, William G. (1977). "cis-Dimethyldiazene". Journal of the American Chemical Society 99 (5): 1661–1663. doi:10.1021/ja00447a072. ISSN 0002-7863.
- ↑ "Azomethane" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/10421#section=Safety-and-Hazards.
- ↑ Francis P. Jahn (September 1937). "The Preparation of Azomethane" (in en). Journal of the American Chemical Society 59 (9): 1761–1762. doi:10.1021/ja01288a502. ISSN 0002-7863. https://pubs.acs.org/doi/abs/10.1021/ja01288a502. Retrieved 2022-10-23.
- ↑ Zhai, Run-Sheng; Chan, Yuet Loy; Chuang, Ping; Hsu, Chien-Kui; Mukherjee, Manabendra; Chuang, Tung J.; Klauser, Ruth (2004-04-01). "Chemisorption and Reaction Characteristics of Methyl Radicals on Cu(110)". Langmuir: The ACS Journal of Surfaces and Colloids 20 (9): 3623–3631. doi:10.1021/la036294u. ISSN 0743-7463. PMID 15875392.
Further reading
- Jianying Zhang, Gangling Chen, Xuedong Gong (September 2021). "Energetic azo compounds based on 2,2′, 4,4′, 6,6′- hexanitroazobenzene: Structures, detonation performance, and sensitivity" (in en). Computational and Theoretical Chemistry 1203: 113344. doi:10.1016/j.comptc.2021.113344. https://linkinghub.elsevier.com/retrieve/pii/S2210271X21002024. Retrieved 2022-10-23.
- Wolfgang W. Schoeller, Guido D. Frey (2016-11-07). "Oxidative Addition of π-Bonds and σ-Bonds to an Al(I) Center: The Second-Order Carbene Property of the AlNacNac Compound" (in en). Inorganic Chemistry 55 (21): 10947–10954. doi:10.1021/acs.inorgchem.6b01488. ISSN 0020-1669. PMID 27739674. https://pubs.acs.org/doi/10.1021/acs.inorgchem.6b01488. Retrieved 2022-10-23.
 |

