Chemistry:Benthiocarb
From HandWiki
| |
| Names | |
|---|---|
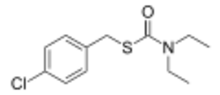
| Preferred IUPAC name
S-[(4-Chlorophenyl)methyl] diethylcarbamothioate | |
| Other names
Thiobencarb, Saturn, Bolero
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C12H16ClNOS | |
| Molar mass | 257.78 g·mol−1 |
| Appearance | Pale yellow to brownish-yellow liquid |
| Density | 1.145-1.180 g cm−3 at 20 °C |
| Melting point | 3.3 °C (37.9 °F; 276.4 K) |
| Boiling point | 126 to 129 °C (259 to 264 °F; 399 to 402 K) at 0.008 Torr |
| 28.0 mg/L at 25 °C | |
| Solubility | Readily soluble in: acetone, ethanol, xylene, methanol, benzene, n-hexane, and acetonitrile |
| log P | 3.42 (octanol/water)[1] |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 165.8 °C (330.4 °F; 438.9 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
Rat, oral 1300 mg/kg
Mouse, oral 560 mg/kg [2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Benthiocarb is a thiocarbamate cholinesterase inhibitor used as an herbicide. Benthiocarb is almost always used to control the weeds around rice crops, but its effectiveness is not specific to just rice crops.[3] The benthiocarb molecule is an organic molecule containing a phenol bonded to a chlorine atom.
See also
References
- ↑ Tomlin, C.D.S., ed (1997). The Pesticide Manual - World Compendium (11th ed.). Surrey, England: British Crop Protection Council. p. 1192.
- ↑ Worthing, C.R. and S.B. Walker, ed (1987). The Pesticide Manual - A World Compendium (8th ed.). Thornton Heath, UK: The British Crop Protection Council. p. 796.
- ↑ United States Environmental Protection Agency (September 1997). "R.E.D. FACTS Thiobencarb". https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/fs_PC-108401_1-Sep-97.pdf.
External links
 |



