Chemistry:Bis(2-chloroethyl)selenide
From HandWiki
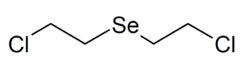
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-Chloro-2-[(2-chloroethyl)selanyl]ethane | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C4H8Cl2Se | |
| Molar mass | 205.98 g·mol−1 |
| Appearance | low-melting colorless solid |
| Melting point | 24 °C (75 °F; 297 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Bis(2-chloroethyl)selenide is the organoselenium compound with the formula Se(CH
2CH
2Cl)
2. As a haloalkyl derivative of selenium, it is an analogue of bis(2-chloroethyl)sulfide, the prototypical sulfur mustard used in chemical warfare. Bis(2-chloroethyl)selenide has not been used as a chemical warfare agent, however it is still a potent alkylating agent and has potential in chemotherapy.[1][2][3][4][5]
See also
References
- ↑ "Linear free energy relationships and cytotoxicities of para-substituted 2-haloethyl aryl selenides and bis(2-chloroethyl) selenides". Journal of Medicinal Chemistry 30 (4): 597–602. April 1987. doi:10.1021/jm00387a003. PMID 3560155.
- ↑ "Phenyl selenones: alkyl transfer by selenium-carbon bond cleavage". Journal of Medicinal Chemistry 33 (6): 1544–7. June 1990. doi:10.1021/jm00168a003. PMID 2342050.
- ↑ "Structure-activity studies on organoselenium alkylating agents". Journal of Pharmaceutical Sciences 79 (1): 57–62. January 1990. doi:10.1002/jps.2600790114. PMID 2313578.
- ↑ "One-Pot Two-Step Approach to Selenides. Phase-Transfer Catalyzed Synthesis of ω-Hydroxyalkyl Selenides.". Synthetic Communications 30 (3): 523–529. 2000. doi:10.1080/00397910008087348.
- ↑ "Synthesis of bis(2-haloethyl) selenides by reaction of selenium dihalides with ethylene.". Russian Journal of Organic Chemistry 50 (2): 291–292. February 2014. doi:10.1134/S1070428014020250.
 |

