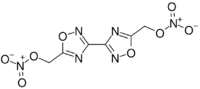
Chemistry:Bis-oxadiazole
| |
| Names | |
|---|---|
| Preferred IUPAC name
[3,3′-Bi-1,2,4-oxadiazole]-5,5′-diylbis(methylene) dinitrate | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| C6H4N6O8 | |
| Molar mass | 288.132 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Bis-oxadiazole, or more formally known as bis(1,2,4-oxadiazole)bis(methylene) dinitrate, is a nitrated heterocyclic compound of the oxadiazole family.[1]
Bis-oxadiazole is related to bis-isoxazole tetranitrate (BITN), which was developed at the United States Army Research Laboratory (ARL). With a high nitrogen content, these compounds are poised to release a large volume of very stable N2.[2] It is a “melt-cast” explosive material that is potentially both more powerful and environmentally friendly alternative to TNT.[3]
Synthesis
Glyoxal condenses with hydroxylamine to yield diaminoglyoxime (DAG). Treating DAG with methyl glycolate in the presence of base at high temperature yields bis(1,2,4-oxadiazole).[4]
Replacement for TNT
TNT is attractive explosive because it is a melt-castable. A low melting point of about 80 °C and high decomposition temperature of 295 °C allows manufacturers to safely pour TNT into molds. The production of TNT generates hazardous waste, e.g. red water and pink water.[1]
Bis-oxadiazole, which is also melt-castable, is about 1.5 times more powerful than TNT and yet produces less hazardous wastes.[1]
| Physical Property | bis-oxadiazole | TNT |
|---|---|---|
| Onset temperature of melting | 84.5 °C | 80.4 °C |
| Onset temperature of decomposition | 183.4 °C | 295.0 °C |
| Derived density from X-ray data | 1.832 g cm−3 | 1.65 g cm−3 |
| Detonation pressure | 29.4 GPa | 20.5 GPa |
| Detonation velocity | 8180 m s−1 | 6950 m s−1 |
| Molar enthalpy of formation | -79.4 kJ mol−1 | -59.3 kJ mol−1 |
A major challenge in the production of bis-oxadiazole is its low yield.[5]
References
- ↑ 1.0 1.1 1.2 Bennett, Jay (July 2, 2018). "So Long TNT, There's a New Explosive in Town". Popular Mechanics. https://www.popularmechanics.com/military/research/a21987787/tnt-replacement-bis-oxadiazole-los-alamos/.
- ↑ McNally, David (May 3, 2016). "Army scientists synthesize high-performing energetic material". Medium. https://medium.com/@RDECOM/army-scientists-synthesize-high-performing-energetic-material-8a5673ff28be.
- ↑ "TNT could be headed for retirement after 116 years on the job". Phys.org. June 14, 2018. https://phys.org/news/2018-06-tnt-years-job.html.
- ↑ 4.0 4.1 Johnson, Eric; Sabatini, Jesse; Chavez, David; Sausa, Rosario; Byrd, Edward; Wingard, Leah; Guzmàn, Pablo (2018). "Bis(1,2,4-oxadiazole)bis(methylene) Dinitrate: A High-Energy Melt-Castable Explosive and Energetic Propellant Plasticizing Ingredient". Organic Process Research & Development 22 (6): 736–740. doi:10.1021/acs.oprd.8b00076.
- ↑ Halford, Bethany (June 5, 2018). "Double oxadiazole could replace TNT". c&en. https://cen.acs.org/materials/Double-oxadiazole-replace-TNT/96/web/2018/06.
 |

