Chemistry:Carbon–nitrogen bond
A carbon–nitrogen bond is a covalent bond between carbon and nitrogen and is one of the most abundant bonds in organic chemistry and biochemistry.[1]
Nitrogen has five valence electrons and in simple amines it is trivalent, with the two remaining electrons forming a lone pair. Through that pair, nitrogen can form an additional bond to hydrogen making it tetravalent and with a positive charge in ammonium salts. Many nitrogen compounds can thus be potentially basic but its degree depends on the configuration: the nitrogen atom in amides is not basic due to delocalization of the lone pair into a double bond and in pyrrole the lone pair is part of an aromatic sextet.
Similar to carbon–carbon bonds, these bonds can form stable double bonds, as in imines; and triple bonds, such as nitriles. Bond lengths range from 147.9 pm for simple amines to 147.5 pm for C-N= compounds such as nitromethane to 135.2 pm for partial double bonds in pyridine to 115.8 pm for triple bonds as in nitriles.[2]
A CN bond is strongly polarized towards nitrogen (the electronegativities of C and N are 2.55 and 3.04, respectively) and subsequently molecular dipole moments can be high: cyanamide 4.27 D, diazomethane 1.5 D, methyl azide 2.17, pyridine 2.19. For this reason many compounds containing CN bonds are water-soluble. N-philes are group of radical molecules which are specifically attracted to the C=N bonds.[3]
Carbon-nitrogen bond can be analyzed by X-ray photoelectron spectroscopy (XPS). Depending on the bonding states the peak positions differ in N1s XPS spectra.[4][5][6]
Nitrogen functional groups
| Chemical class | Bond order | Formula | Structural Formula | Example | Avg. C–N bond length (Å)[7] |
|---|---|---|---|---|---|
| Amines | 1 | R2C-NH2 | 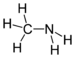 Methylamine |
1.469 (neutral amine) 1.499 (ammonium salt) | |
| Aziridines | 1 | CH2NHCH2 | 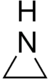
|
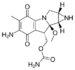 Mitomycin |
1.472 |
| Azides | 1 | R2C-N3 | Phenyl azide | ||
| Anilines | 1 | Ph-NH2 | 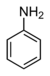
|
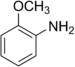 Anisidine |
1.355 (sp2 N) 1.395 (sp3 N) 1.465 (ammonium salt) |
| Pyrroles | 1 | 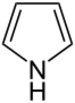
|
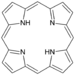 Porphyrin |
1.372 | |
| Amides | 1.2 | R-CO-NR2 | 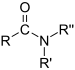
|
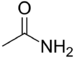 Acetamide |
1.325 (primary) 1.334 (secondary) 1.346 (tertiary) |
| Pyridines | 1.5 | pyr | 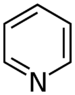
|
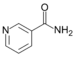 Nicotinamide |
1.337 |
| Imines | 2 | R2C=NR | 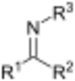
|
 DBN |
1.279 (C=N bond) 1.465 (C–N bond) |
| Nitriles | 3 | R-CN |  Benzonitrile |
1.136 | |
| Isonitriles | 3 | R-NC | TOSMIC |
1.154[8] |
See also
- Cyanide
- Other carbon bonds with group 15 elements: carbon–nitrogen bonds, carbon–phosphorus bonds
- Other carbon bonds with period 2 elements: carbon–lithium bonds, carbon–beryllium bonds, carbon–boron bonds, carbon–carbon bonds, carbon–nitrogen bonds, carbon–oxygen bonds, carbon–fluorine bonds
- Carbon–hydrogen bond
References
- ↑ Organic Chemistry John McMurry 2nd Ed.
- ↑ CRC Handbook of Chemistry and Physics 65Th Ed.
- ↑ Falzon, Chantal T.; Ryu, Ilhyong; Schiesser, Carl H. (2002). "5-Azahexenoyl radicals cyclize via nucleophilic addition to the acyl carbon rather than 5-exo homolytic addition at the imine". Chemical Communications (20): 2338–9. doi:10.1039/B207729A. PMID 12430429.
- ↑ Kato, Tomofumi; Yamada, Yasuhiro; Nishikawa, Yasushi; Otomo, Toshiya; Sato, Hayato; Sato, Satoshi (2021-07-12). "Origins of peaks of graphitic and pyrrolic nitrogen in N1s X-ray photoelectron spectra of carbon materials: quaternary nitrogen, tertiary amine, or secondary amine?" (in en). Journal of Materials Science 56 (28): 15798–15811. doi:10.1007/s10853-021-06283-5. ISSN 1573-4803. https://doi.org/10.1007/s10853-021-06283-5.
- ↑ Yamada, Yasuhiro; Kim, Jungpil; Matsuo, Shintaro; Sato, Satoshi (2014-04-01). "Nitrogen-containing graphene analyzed by X-ray photoelectron spectroscopy" (in en). Carbon 70: 59–74. doi:10.1016/j.carbon.2013.12.061. ISSN 0008-6223. https://www.sciencedirect.com/science/article/abs/pii/S0008622313012165.
- ↑ Yamada, Yasuhiro; Tanaka, Haruki; Kubo, Shingo; Sato, Satoshi (2021-09-01). "Unveiling Bonding States and Roles of Edges in Nitrogen-Doped Graphene Nanoribbon by X-ray Photoelectron Spectroscopy" (in en). Carbon 185: 342–367. doi:10.1016/j.carbon.2021.08.085. ISSN 0008-6223. https://www.sciencedirect.com/science/article/abs/pii/S000862232100885X.
- ↑ F. H. Allen, O. Kennard, D. G. Watson, L. Brammer, A. G. Orpen. Tables of bond Lengths determined by X-Ray and Neutron Diffraction. Part 1. Bond Lengths in Organic Compounds. J. Chem. Soc. Perkin Trans. II 1987, S1-S19.
- ↑ Bano, Huma; Yousuf, Sammer (2015). "Crystal structure of p-toluenesulfonylmethyl isocyanide". Acta Crystallogr. E 71 (6): o412. doi:10.1107/S2056989015008816. PMID 26090196.
 |
