Chemistry:Cedrol
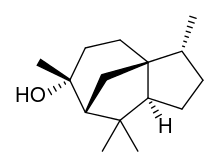
| |
| Names | |
|---|---|
| IUPAC name
8α-Cedran-8β-ol
| |
| Systematic IUPAC name
(3R,3aS,6R,7R,8aS)-3,6,8,8-Tetramethyloctahydro-1H-3a,7-methanoazulen-6-ol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C15H26O | |
| Molar mass | 222.372 g·mol−1 |
| Density | 1.01 g/mL |
| Melting point | 86 to 87 °C (187 to 189 °F; 359 to 360 K)[1] |
| Boiling point | 273 °C (523 °F; 546 K)[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cedrol is a sesquiterpene alcohol found in the essential oil of conifers (cedar oil), especially in the genera Cupressus (cypress) and Juniperus (juniper). It has also been identified in Origanum onites, a plant related to oregano.[3] Its main uses are in the chemistry of aroma compounds.[4] It makes up about 19% of cedarwood oil Texas and 15.8% of cedarwood oil Virginia.[5]
Cedrol has not been proven to be toxic in humans. It has been shown to have antioxidant and antiinflammatory along with other beneficial effects. In skin sensitization tests 2/20 people showed negative effects, and on the second test there was no sensitivity found. This compound and ones similar have been found to have antiseptic, anti-inflammatory, antispasmodic, tonic, astringent, diuretic, sedative, insecticidal, and antifungal activities in vitro.[6] These compounds are used globally in traditional medicine and cosmetics.[7] Results of a 2015 study suggest that cedrol strongly attracts pregnant female mosquitoes after they have fed, which can be used to create cedrol-baited traps.[8]
See also
- Cedrene, another component of cedar oil
References
- ↑ Budavari, Susan, ed. (1996), An Encyclopedia of Chemicals, Drugs, and Biologicals (12th ed.), Merck, ISBN 0911910123, 1961
- ↑ Template:Aldrich
- ↑ Connolly, JD, ed (1991). Dictionary of Terpenoids. 1 Mono- and sesquiterpenoids. Chapman&Hall. SQ02555. ISBN 0-412-25770-X.
- ↑ Breitmeier, E (2006). Terpenes: flavors, fragrances, pharmaca, pheromones. Wiley-VCH. pp. 46–47. ISBN 3-527-31786-4.
- ↑ Susan Barclay-Nichols. "Point of Interest!". swiftcraftymonkey.blogspot.com. http://swiftcraftymonkey.blogspot.com/2012/02/essential-oils-cedarwood-science-of.html.
- ↑ Jeong, H. U.; Kwon, S. S.; Kong, T. Y.; Kim, J. H.; Lee, H. S. (2014). "Inhibitory effects of cedrol, β-cedrene, and thujopsene on cytochrome P450 enzyme activities in human liver microsomes". Journal of Toxicology and Environmental Health. Part A 77 (22–24): 1522–32. doi:10.1080/15287394.2014.955906. PMID 25343299.
- ↑ "Cedrol". TOXNET: Toxicology Data Network. https://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+8265.
- ↑ Lindh, Jenny; Okal, Michael (March 2015). "Discovery of an oviposition attractant for gravid malaria vectors of the Anopheles gambiae species complex". Malaria Journal 14 (119): 119. doi:10.1186/s12936-015-0636-0. PMID 25885703. PMC 4404675. http://www.sciguru.org/newsitem/18734/cedrol-naturally-occurring-bait-malaria-spreading-pregnant-mosquitoes.
 |
