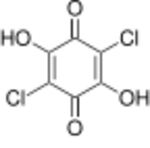
Chemistry:Chloranilic acid
From HandWiki
| |
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
2,5-Dichloro-3,6-dihydroxycyclohexa-2,5-diene-1,4-dione | |
| Other names
2,5-Dichloro-3,6-dihydroxy-1,4-benzoquinone
2,5-Dichloro-3,6-dihydroxybenzoquinone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H2Cl2O4 | |
| Molar mass | 208.98 g/mol |
| Appearance | orange or red crystals or powder |
| Density | 1.96 g/cm3[2] |
| Melting point | ≥300 °C |
| Acidity (pKa) | 2.95, 4.97[3] |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
| Flash point | 135.4 °C (275.7 °F; 408.5 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Chloranilic acid is an organic compound with the chemical formula C
6Cl
2O
2(OH)
2. It is a red-orange solid. The compound is obtained by hydrolysis of chloranil:
- C
6Cl
4O
2 + 2 H
2O → C
6Cl
2O
2(OH)
2 + 2 HCl
It is centrosymmetric, planar molecule. It also crystallizes as a dihydrate.[2]
Chloranilic acid is a noteworthy hydroxyquinone that is somewhat acidic owing to the presence of the two chloride substituents. The conjugate base, C6H2Cl2O4]2- readily forms coordination complexes often linking pairs of many metal ions.[3]
See also
References
- ↑ "Chloranilic acid". Sigma-Aldrich. http://www.sigmaaldrich.com/catalog/product/aldrich/c8136?lang=en®ion=US.
- ↑ 2.0 2.1 Andersen, E. K. (1967). "The Crystal and Molecular Structure of Hydroxyquinones and Salts of Hydroxyquinones. I. Chloranilic Acid". Acta Crystallographica 22 (2): 188–191. doi:10.1107/S0365110X67000325.
- ↑ 3.0 3.1 Mostafa, S. I. (1999). "Complexes of 2,5-Dihydroxy-1,4-Benzoquinone and Chloranilic Acid with Second and Third Row Transition Elements". Transition Metal Chemistry 24 (3): 306–310. doi:10.1023/A:1006944124791.
 |
