Chemistry:Chlorine oxide
From HandWiki
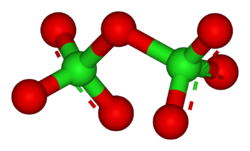
2O
7
Chlorine and oxygen can bond in many ways:
- chlorine monoxide radical, ClO•, chlorine (II) oxide radical
- chloroperoxyl radical, ClOO•, chlorine (II) peroxide radical
- chlorine dioxide, ClO
2, chlorine (IV) oxide - chlorine trioxide radical, ClO
3•, chlorine (VI) oxide radical - chlorine tetroxide radical, ClO
4•, chlorine (VII) oxide radical - dichlorine monoxide, Cl
2O, chlorine (I) oxide - chlorine peroxide, Cl
2O
2, dimer of chlorine monoxide radical or ClO dimer, chlorine (I) peroxide- chloryl chloride, ClO
2Cl, chlorine (0,IV) oxide - chlorine chlorite, ClOClO, chlorine (I,III) oxide
- chloryl chloride, ClO
- dichlorine trioxide, Cl
2O
3 as O–Cl–ClO
2, chlorine (III,V) oxide- dichlorine trioxide, Cl
2O
3 as possible isomer Cl–O–ClO
2, chlorine (I,V) oxide - dichlorine trioxide, Cl
2O
3 as hypothetical isomer O–Cl–O–Cl–O, chlorine (III) oxide
- dichlorine trioxide, Cl
- dichlorine tetroxide, also known as chlorine perchlorate, Cl
2O
4 or ClOClO
3, chlorine (I,VII) oxide - dichlorine pentoxide, Cl
2O
5 or ClOOClO
3, is hypothetical - dichlorine hexoxide or chloryl perchlorate, Cl
2O
6 or [ClO
2]+
[ClO
4]−
, chlorine (V,VII) oxide - dichlorine heptoxide, Cl
2O
7, chlorine (VII) oxide - dichlorine octoxide, chlorine (VII) oxide peroxide or dimer of chlorine tetroxide radical, Cl
2O
8 or (OClO
3)
2
Several ions are also chlorine oxides:
- chloryl, ClO+
2 - perchloryl, ClO+
3 - hypochlorite, ClO−
- chlorite, ClO−
2 - chlorate, ClO−
3 - perchlorate, ClO−
4
See also
- Oxygen fluoride(s), bromine oxide(s), iodine oxide(s) – analogous oxygen halide and halogen oxides
- Sulfur fluoride(s), sulfur chloride(s), sulfur bromide(s), sulfur iodide(s) – analogous sulfur halides, some of which are valence isoelectronic with chlorine oxides.
References
 |
