Chemistry:Chloroformic acid
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Carbonochloridic acid[1] | |||
| Other names
Chloroformic acid
Chlorocarbonic acid | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| ClCO 2H | |||
| Molar mass | 80.47 g·mol−1 | ||
| Acidity (pKa) | 0.27[2] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
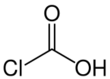
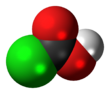
Chloroformic acid is a chemical compound with the formula ClCO
2H. It is the single acyl-halide derivative of carbonic acid (phosgene is the double acyl-halide derivative). Chloroformic acid is also structurally related to formic acid, in a way that the non-acidic hydrogen of formic acid is replaced by chlorine. Despite the similar name, it is very different from chloroform. It is described as unstable.[3]
Chloroformic acid itself is too unstable to be handled for chemical reactions. However, many esters of this carboxylic acid are stable and these chloroformates are important reagents in organic chemistry.[4] They are used to prepare mixed carboxylic acid anhydrides used in peptide synthesis. Important chloroformate esters include 4-nitrophenyl chloroformate, fluorenylmethyloxycarbonylchloride, benzyl chloroformate and ethyl chloroformate.
See also
References
- ↑ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. pp. 776–777. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ↑ "Archived copy". https://hmdb.ca/metabolites/HMDB0250109.
- ↑ Gibson, Harry W. (1969). "Chemistry of Formic Acid and Its Simple Derivatives". Chemical Reviews 69 (5): 673–692. doi:10.1021/cr60261a005.
- ↑ Matzner, Markus; Kurkjy, Raymond P.; Cotter, Robert J. (1964). "The Chemistry of Chloroformates". Chemical Reviews 64 (6): 645–687. doi:10.1021/cr60232a004.
 |