Chemistry:Cis-3-Hexenal
From HandWiki
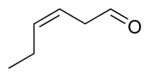
| |
| Names | |
|---|---|
| Preferred IUPAC name
(3Z)-Hex-3-enal | |
| Other names
(Z)-Hex-3-enal
cis-3-Hexenal Leaf aldehyde | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H10O | |
| Molar mass | 98.145 g·mol−1 |
| Density | 0.851 g/cm3 |
| Boiling point | 126 °C (259 °F; 399 K) |
| Related compounds | |
Related alkenals
|
Acrolein Crotonaldehyde |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
cis-3-Hexenal, also known as (Z)-3-hexenal and leaf aldehyde, is an organic compound with the formula CH3CH2CH=CHCH2CHO. It is classified as an unsaturated aldehyde. It is a colorless liquid and an aroma compound with an intense odor of freshly cut grass and leaves.[1][2]
Occurrence
It is one of the major volatile compounds in ripe tomatoes, although it tends to isomerize into the conjugated trans-2-hexenal.[3] It is produced in small amounts by most plants and it acts as an attractant to many predatory insects. It is also a pheromone in many insect species.[4]

See also
- cis-3-hexen-1-ol has a similar but weaker odor and is used in flavors and perfumes.
- 1-Hexanol, another volatile organic compound, also considered responsible for the freshly mowed grass odor
External links
References
- ↑ Cotton, Simon (2017). Molecule of the Month: Hexenal. Chm.bris.ac.uk. doi:10.6084/m9.figshare.5245834. http://www.chm.bris.ac.uk/motm/hexenal/hexenalh.htm. Retrieved 2018-07-26.
- ↑ Hexenal / Chemistry World, Royal Society of Chemistry, 27 November 2013
- ↑ Buttery, Ron G.; Teranishi, Roy; Ling, Louisa C. (1987). "Fresh tomato aroma volatiles: A quantitative study". Journal of Agricultural and Food Chemistry 35 (4): 540–544. doi:10.1021/jf00076a025.
- ↑ Ashraf El-Sayed. "Pheromone database". Pherobase.com. http://www.pherobase.com/database/compound/compounds-detail-Z3-6Ald.php. Retrieved 2018-07-26.
- ↑ KenjiMatsui (2006). "Green leaf volatiles: hydroperoxide lyase pathway of oxylipin metabolism". Current Opinion in Plant Biology 9 (3): 274–280. doi:10.1016/j.pbi.2006.03.002. PMID 16595187.
 |
