Chemistry:Curzerene
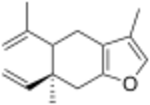
| |
| Names | |
|---|---|
| IUPAC name
(6S)-6-Ethenyl-3,6-dimethyl-5-prop-1-en-2-yl-5,7-dihydro-4H-1-benzofuran
| |
| Other names
Isogermafuren
Isofuranogermacrene | |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| C15H20O | |
| Molar mass | 216.324 g·mol−1 |
| Melting point | 65.3 °C estimated |
| Boiling point | 282.8±40.0 °C estimated |
| Poorly soluble in water | |
| Hazards | |
| Flash point | 117.50 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Curzerene is a volatile, aromatic terpenoid found in many herbs and spices, such as Curcuma zeodaria.[2] It is bioactive isolate of Caribbean corals[3] and is also found in myrrh.[4] More specifically it has been found to make up a significant portion - 12.97% - of the smoke produced from burning Commiphora myrrha oleo gum resin.[5] It is also a major component of myrrh oil, which has been shown in vitro to possess anti-inflammatory properties at sub-toxic by inhibiting the production of the inflammatory cytokine IL-6 by human gingival fibroblasts. Anecdotal evidence exists to support the anti-inflammatory effect of myrrh oil.[6]
Curzerene represents 13.7% of the essential oil extracted from Smyrnium olusatrum, which has demonstrated significant antimicrobial activity in vitro.[7]
References
- ↑ "KNApSAcK Metabolite Information - C00012040". http://www.knapsackfamily.com/knapsack_core/information.php?word=C00012040.
- ↑ PubChem. "Curzerene" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/572766.
- ↑ Roussis, V; Vagias, C; Tsitsimpikou, C; Diamantopoulou, N (2000). "Chemical variability of the volatile metabolites from the Caribbean corals of the genus Gorgonia". Zeitschrift für Naturforschung C 55 (5–6): 431–41. doi:10.1515/znc-2000-5-620. PMID 10928556.
- ↑ Hanus, Lumir O.; Rezanka, Tomas; Dembitsky, Valery M.; Moussaieff, Arieh (2005). "Myrrh - Commiphora Chemistry". Biomedical Papers 149 (1): 3–28. doi:10.5507/bp.2005.001. PMID 16170385.
- ↑ Ljaljević Grbić, Milica; Unković, Nikola; Dimkić, Ivica; Janaćković, Peđa; Gavrilović, Milan; Stanojević, Olja; Stupar, Miloš; Vujisić, Ljubodrag et al. (June 2018). "Frankincense and myrrh essential oils and burn incense fume against micro-inhabitants of sacral ambients. Wisdom of the ancients?". Journal of Ethnopharmacology 219: 1–14. doi:10.1016/j.jep.2018.03.003. ISSN 0378-8741. PMID 29530608.
- ↑ Tipton, D.A.; Hamman, N.R.; Dabbous, M.Kh. (March 2006). "Effect of myrrh oil on IL-1β stimulation of NF-κB activation and PGE2 production in human gingival fibroblasts and epithelial cells". Toxicology in Vitro 20 (2): 248–255. doi:10.1016/j.tiv.2005.07.004. ISSN 0887-2333. PMID 16112536.
- ↑ Daroui-Mokaddem, Habiba; Kabouche, Ahmed; Bouacha, Mabrouka; Soumati, Boudjemaa; El-Azzouny, Aida; Bruneau, Christian; Kabouche, Zahia (October 2010). "GC/MS Analysis and Antimicrobial Activity of the Essential Oil of Fresh Leaves of Eucalytus Globulus, and Leaves and Stems of Smyrnium Olusatrum from Constantine (Algeria)". Natural Product Communications 5 (10): 1934578X1000501. doi:10.1177/1934578x1000501031. ISSN 1934-578X.
 |

