Chemistry:Cyanomethine
| |
| Names | |
|---|---|
| IUPAC name
4-Amino-2,6-dimethylpyrimidine
| |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C6H9N3 | |
| Molar mass | 123.159 g·mol−1 |
| Appearance | crystalline solid |
| Odor | irritating[1] |
| Melting point | 180–185 °C (356–365 °F; 453–458 K) |
| soluble | |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P264+265Script error: No such module "Preview warning".Category:GHS errors, P271, P280, P302+352, P304+340, P305+351+338, P319Script error: No such module "Preview warning".Category:GHS errors, P321, P332+317Script error: No such module "Preview warning".Category:GHS errors, P337+317Script error: No such module "Preview warning".Category:GHS errors, P362+364Script error: No such module "Preview warning".Category:GHS errors, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
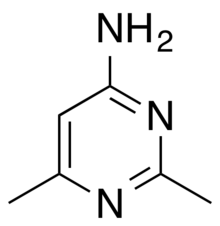
Cyanomethine (4-amino-2,6-dimethylpyrimidine) is an amino and methylated derivative of pyrimidine with the chemical formula C
6H
9N
3, belonging to a class named cyanalkines.[2]
Properties and synthesis
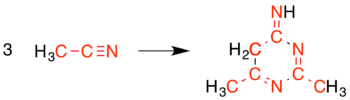
Cyanomethine is a strongly basic[4] colourless to white crystalline solid. It is soluble in water and slightly soluble in alcohol.[1] It is prepared by the trimerisation of acetonitrile with sodium or potassium,[5] with the corresponding metal cyanide and C
4H
6N
2 (possibly 2-methylimidazole or 3-methylpyrazole; iminoacetonitrile has been identified[6]) as the main byproducts.[7] It can also made by reaction of sodium methoxide and acetonitrile.[2]
The correspondence of the three acetonitrile units to a tautomer of cyanomethine is:
At higher pressure, sodium methoxide instead catalyzes trimerization to form 2,4,6-Trimethyl-1,3,5-triazine.[8]
2,6-Diethyl-5-methyl-4-pyrimidinamine (cyan(o)ethine) is an analogous structure that can be made by trimerization of propionitrile.[2]
Cyanomethine can form complexes with platinum(II) and platinum(IV) compounds.[9]
References
- ↑ 1.0 1.1 1.2 Roscoe, H. E. (1881). A Treatise on chemistry volume 3, 1881.page 523-524
- ↑ 2.0 2.1 2.2 2.3 Polymerisation of Nitriles, Journal of the Chemical Society. (1890). United Kingdom: Chemical Society.page 1158
- ↑ "4-Amino-2,6-dimethylpyrimidine" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/68039#section=Safety-and-Hazards.
- ↑ Karrer, P. (1950). Organic Chemistry. page 819
- ↑ Miller, W. A., McLeod, H. (1880). Elements of Chemistry: Theoretical and Practical, page 174
- ↑ Derivatives of Hydrocyanic Acid. Competition Science Vision. Jan 2001 page 1494
- ↑ Methyl cyanide, Thorpe, T. E. (1895). A Dictionary of Applied Chemistry. United Kingdom: Longmans. page 575
- ↑ T. L. Cairns, A. W. Larchar, and B. C. McKusick, The Trimerization of Nitriles at High Pressures, J. Am. Chem. Soc. 1952, 74, 22, 5633–5636 Publication Date: November 1, 1952 [1]
- ↑ Kovala-Demertzi, D. Platinum(II) and platinum(IV) complexes of 2-amino-4,6-dimethylpyrimidine. Transition Met Chem 15, 23–26 (1990). https://doi.org/10.1007/BF01032225
 |