Chemistry:Cyclobutanone
| |
| Names | |
|---|---|
| Preferred IUPAC name
Cyclobutanone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C4H6O | |
| Molar mass | 70.091 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.9547 g/cm3 (0 °C)[1] |
| Melting point | −50.9 °C (−59.6 °F; 222.2 K)[1] |
| Boiling point | 99.75 °C (211.55 °F; 372.90 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
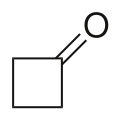
Cyclobutanone is an organic compound with molecular formula (CH2)3CO. It is a four-membered cyclic ketone (cycloalkanone). It is a colorless volatile liquid at room temperature. Since cyclopropanone is highly sensitive, cyclobutanone is the smallest easily handled cyclic ketone.
Preparation

The Russian chemist Nikolai Kischner first prepared cyclobutanone in a low yield from cyclobutanecarboxylic acid.[2][3] Kischner's process, involving several steps, is cumbersome and inefficient; more efficient, high-yielding syntheses have since been developed.[4]
One strategy involves degradation of five-carbon building blocks. For example, the oxidative decarboxylation of cyclobutanecarboxylic acid was improved by the use of other reagents and methods.
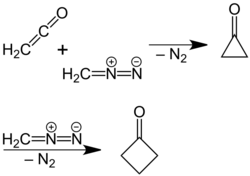
A newer, more efficient preparation of cyclobutanone was found by P. Lipp and R. Köster in which a solution of diazomethane in diethyl ether is reacted with ketene.[5] This reaction is based on a ring expansion of the cyclopropanone intermediate initially formed, wherein molecular nitrogen is split off:
The reaction mechanism was confirmed by a reaction using 14C-labeled diazomethane.[6]
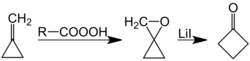
Another synthesis of cyclobutanone involves lithium-catalyzed rearrangement of oxaspiropentane, which is formed by epoxidation of the easily accessible methylenecyclopropane:[7][8]
Cyclobutanone can also be prepared in a two step procedure by dialkylation of 1,3-dithiane with 1-bromo-3-chloropropane followed by deprotection to the ketone with mercuric chloride (HgCl2) and cadmium carbonate (CdCO3).[9]
Reactions
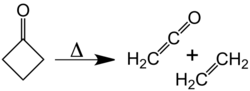
At about 350 °C, cyclobutanone decomposes into ethylene and ketene.[10] The activation energy for this [2+2] cycloelimination is 52 kcal/mol. The reverse reaction, the [2+2] cycloaddition of ketene and ethylene, has never been observed.
See also
Other cyclic ketones:
References
- ↑ 1.0 1.1 1.2 CRC Handbook of Chemistry and Physics. 90. Boca Raton, Florida: CRC Press.
- ↑ N. Kishner (1905). "'Über die Einwirkung von Brom auf die Amide α-bromsubstituierter Säuren". Journal der Russischen Physikalisch-Chemischen Gesellschaft 37: 103–105.
- ↑ N. Kishner (1905). "Über das Cyklobutanon". Journal der Russischen Physikalisch-Chemischen Gesellschaft 37: 106–109.
- ↑ Dieter Seebach (1971). "Isocyclische Vierringverbindungen". in Houben. Methoden der Organischen Chemie. IV/4. Stuttgart: Georg Thieme Verlag.
- ↑ P. Lipp und R. Köster (1931). "Ein neuer Weg zum Cyclobutanon". Berichte der Deutschen Chemischen Gesellschaft 64 (11): 2823–2825. doi:10.1002/cber.19310641112.
- ↑ Semenow, Dorothy A.; Cox, Eugene F.; Roberts, John D. (1956). "Small-Ring Compounds. XIV. Radioactive Cyclobutanone from Ketene and Diazomethane-14C1". Journal of the American Chemical Society 78 (13): 3221–3223. doi:10.1021/ja01594a069.
- ↑ Salaün, J. R.; Conia, J. M. (1971). "Oxaspiropentane. A rapid route to cyclobutanone". Journal of the Chemical Society D: Chemical Communications (23): 1579b–1580. doi:10.1039/C2971001579B.
- ↑ J. R. Salaün, J. Champion, J. M. Conia (1977). "Cyclobutanone from Methylenecyclopropane via Oxaspiropentane". Organic Syntheses 57: 36. doi:10.15227/orgsyn.057.0036. http://www.orgsyn.org/demo.aspx?prep=CV6P0320.; Collective Volume, 6, pp. 320
- ↑ D. Seebach, A. K. Beck (1971). "Cyclic Ketones from 1,3-Dithiane: Cyclobutanone". Organic Syntheses 51: 76. doi:10.15227/orgsyn.051.0076. http://www.orgsyn.org/demo.aspx?prep=CV6P0316.; Collective Volume, 6, pp. 316
- ↑ Das, M. N.; Kern, F.; Coyle, T. D.; Walters, W. D. (1954). "The Thermal Decomposition of Cyclobutanone1". Journal of the American Chemical Society 76 (24): 6271–6274. doi:10.1021/ja01653a013.
 |