Chemistry:DHSA
| |
| Names | |
|---|---|
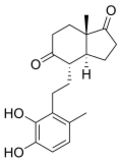
| IUPAC name
3,4-Dihydroxy-9,10-secoandrosta-1,3,5(10)-triene-9,17-dione
| |
| Systematic IUPAC name
(3aS,4S,7aS)-4-[2-(2,3-Dihydroxy-6-methylphenyl)ethyl]-7a-methylhexahydro-1H-indene-1,5(4H)-dione | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
PubChem CID
|
|
| |
| |
| Properties | |
| C19H24O4 | |
| Molar mass | 316.39146 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
3,4-DHSA is an organic compound which is the intermediate product of the metabolism of cholesterol, by the bacteria most commonly responsible for tuberculosis (Mycobacterium tuberculosis).[1] 3,4-DHSA is an acronym for 3,4-dihydroxy-9,10-seco-androst-1,3,5(10)-triene-9,17-dione, the official name of this substance. It is classified as a secosteroid, since one of the four rings of cholesterol from which it is derived is broken.
3,4-DHSA is a catecholic intermediate (a compound containing an aromatic ring with two adjacent hydroxyl groups) produced by M. tuberculosis during the breakdown of cholesterol.[1] 3,4-DHSA is also produced by other bacteria such as Comamonas testosteroni.[2][3]
A particular type of enzyme known as extradiol dioxygenase is responsible for the oxidation and ring opening of 3,4-DHSA to 4,9-DSHA (see metabolic scheme below). M. tuberculosis bacteria that are deficient in this enzyme are less lethal than wild-type bacteria. 3,4-DHSA itself appears to be toxic to the bacteria while the breakdown products of 3,4-DHSA can be used as energy source by the bacteria. Hence blocking the oxidation of 3,4-DHSA by the extradiol dioxygenase enzyme may be useful in the treatment of tuberculosis.[1]
A crystal structure of DHSA in complex with M. tuberculosis iron-dependent extradiol dioxygenase has been determined.[1]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 PDB: 2ZI8; Ramakrishnan, Lalita, ed (March 2009). "Studies of a Ring-Cleaving Dioxygenase Illuminate the Role of Cholesterol Metabolism in the Pathogenesis of Mycobacterium tuberculosis". PLOS Pathog. 5 (3). doi:10.1371/journal.ppat.1000344. PMID 19300498.
- ↑ "Steroid degradation gene cluster of Comamonas testosteroni consisting of 18 putative genes from meta-cleavage enzyme gene tesB to regulator gene tesR". Biochem. Biophys. Res. Commun. 324 (2): 597–604. November 2004. doi:10.1016/j.bbrc.2004.09.096. PMID 15474469.
- ↑ "The genes encoding the hydroxylase of 3-hydroxy-9,10-secoandrosta-1,3,5(10)-triene-9,17-dione in steroid degradation in Comamonas testosteroni TA441". J. Steroid Biochem. Mol. Biol. 92 (3): 143–54. October 2004. doi:10.1016/j.jsbmb.2004.09.002. PMID 15555908.
 |
