Chemistry:DPEphos
From HandWiki
Short description: Chemical compound
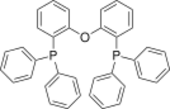
| |
| Names | |
|---|---|
| Preferred IUPAC name
[Oxydi(2,1-phenylene)]bis(diphenylphosphane) | |
| Other names
DPEphos, Bis[(2-diphenylphosphino)phenyl] ether
| |
| Identifiers | |
PubChem CID
|
|
| Properties | |
| C36H28OP2 | |
| Molar mass | 538.567 g·mol−1 |
| Appearance | white powder |
| Melting point | 175 - 176 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Bis[(2-diphenylphosphino)phenyl] ether, also known as DPEphos, is a wide bite angle diphosphine ligand used in inorganic and organometallic chemistry. The name DPEphos is derived from diphenyl ether (DPE) which makes up the ligand's backbone. It is similar to Xantphos, another diphosphine ligand, but is more flexible and has a smaller bite angle (104 vs 108°).[1] It is synthesized from chlorodiphenylphosphine and DPE.[2]
References
- ↑ Birkholz (née Gensow), Mandy-Nicole; Freixa, Zoraida; van Leeuwen, Piet W. N. M. (2009). "Bite angle effects of diphosphines in C–C and C–X bond forming cross coupling reactions". Chemical Society Reviews 38 (4): 1099–118. doi:10.1039/B806211K. PMID 19421583.
- ↑ Kranenburg, Mirko; van der Burgt, Yuri E. M.; Kamer, Paul C. J.; van Leeuwen, Piet W. N. M.; Goubitz, Kees; Fraanje, Jan (June 1995). "New Diphosphine Ligands Based on Heterocyclic Aromatics Inducing Very High Regioselectivity in Rhodium-Catalyzed Hydroformylation: Effect of the Bite Angle". Organometallics 14 (6): 3081–3089. doi:10.1021/om00006a057. https://pure.uva.nl/ws/files/2969936/882_9954y.pdf.
 |
