Chemistry:Darusentan
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Elimination half-life | 12.5 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
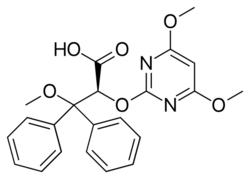
| Formula | C22H22N2O6 |
| Molar mass | 410.426 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Darusentan (LU-135252; HMR-4005) is an endothelin receptor antagonist.[1] Gilead Colorado, a subsidiary of Gilead Sciences,[2] under license from Abbott Laboratories, is developing darusentan for the potential treatment of uncontrolled hypertension.
In June 2003, Myogen licensed the compound from Abbott for its application in the cancer field.[3]
In May 2007, a randomized, double-blind, active control, parallel assignment, safety and efficacy phase III trial was initiated in subjects who had completed the maintenance period of the DAR-312 study, but was terminated because the study did not reach its primary endpoints.[4]
See also
References
- ↑ "Darusentan, a selective endothelin A receptor antagonist, for the oral treatment of resistant hypertension". Therapeutic Advances in Cardiovascular Disease 4 (4): 231–40. August 2010. doi:10.1177/1753944710373785. PMID 20660536. https://www.zora.uzh.ch/id/eprint/36404/2/Enseleit_2010_4.pdf.
- ↑ Gilead Sciences[yes|permanent dead link|dead link}}]
- ↑ "Darusentan - Gilead Sciences". Adis Insight. Springer Nature Switzerland AG. https://adisinsight.springer.com/drugs/800007664.
- ↑ Clinical trial number NCT00389675 for "DORADO-AC-EX - A Long-Term Safety Extension Study to the Phase 3 DORADOC-AC Study (Protocol DAR-312) of Darusentan in Resistant Hypertension (Darusentan)" at ClinicalTrials.gov
 |
