Chemistry:Ambrisentan
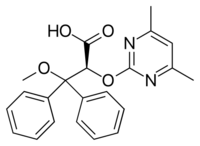 | |
| Clinical data | |
|---|---|
| Trade names | Letairis, Volibris, Pulmonext |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a612023 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 99% |
| Elimination half-life | 15 hours (terminal) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C22H22N2O4 |
| Molar mass | 378.428 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Ambrisentan, sold under the brand name Letairis among others, is a drug used for the treatment of pulmonary hypertension.[1][3] It is an endothelin receptor antagonist.[1]
The peptide endothelin constricts muscles in blood vessels, increasing blood pressure. Ambrisentan, which relaxes those muscles, is an endothelin receptor antagonist, and is selective for the type A endothelin receptor (ETA).[4] Ambrisentan significantly improved exercise capacity (6-minute walk distance) compared with placebo in two double-blind, multicenter trials (ARIES-1 and ARIES-2).[5] Like all endothelin receptor antagonists, Ambrisentan is contraindicated in pregnant women as well as those who are trying to become pregnant, due to the potential for teratogenic effects on the fetus.[6]
Ambrisentan was approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA), and designated an orphan drug, for the treatment of pulmonary hypertension.[7][2][8][9]
Medical uses
Ambrisentan is indicated for the treatment of pulmonary arterial hypertension (WHO Group 1) in people with WHO class II or III symptoms to improve exercise capacity and delay clinical worsening.[1][2]
Mechanism of action
Ambrisentan is a drug that blocks endothelin, an endogenous hormone found in higher quantities in patients with pulmonary arterial hypertension. Endothelin binds to two receptors, ETA and ETB. ETA is responsible for cell growth in the vessels as well as vasoconstriction, while ETB plays a role in vasodilation, endothelin 1 clearance, and antiproliferation of cells.
Birth defects
Endothelin receptor activation mediates strong pulmonary vasoconstriction and positive inotropic effect on the heart. These physiologic effects are vital for the development of the fetal cardiopulmonary system. In addition to this, endothelin receptors are also known to play a role in neural crest cell migration, growth, and differentiation. As such, endothelin receptor antagonists such as ambrisentan are known to be teratogenic.
Society and culture
Brand names
Ambrisentan is sold under the brand name Letairis,[1] Volibris,[2] and Pulmonext.[citation needed]
Publications
|
Last updated 9/2/2015 | |
|---|---|
| 8/15/2015 Reprod. Toxicol. | Endothelin receptor activation mediates strong pulmonary vasoconstriction and positive inotropic effect on the heart. These physiologic effects are vital for the development of the fetal cardiopulmonary system. As such, endothelin receptor antagonists such as ambrisentan are teratogenic.[10] |
| 8/27/2015 NEJM | Ambrisentan when used in combination therapy with tadalafil was found to be more efficacious in treating treatment naive patients with WHO class II or III pulmonary arterial hypertension than monotherapy using either drug.[11] |
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "Letairis- ambrisentan tablet, film coated". 4 September 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=725d4e73-6c83-477a-adc6-0ae4a133a844.
- ↑ 2.0 2.1 2.2 2.3 "Volibris EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/volibris.
- ↑ "Ambrisentan Monograph for Professionals". American Society of Health-System Pharmacists. 7 January 2019. https://www.drugs.com/monograph/ambrisentan.html.
- ↑ "Ambrisentan, a non-peptide endothelin receptor antagonist". Cardiovascular Drug Reviews 24 (1): 63–76. 2006. doi:10.1111/j.1527-3466.2006.00063.x. PMID 16939634.
- ↑ "Ambrisentan". American Journal of Cardiovascular Drugs 11 (4): 215–26. August 2011. doi:10.2165/11207340-000000000-00000. PMID 21623643.
- ↑ "Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2". Circulation 117 (23): 3010–9. June 2008. doi:10.1161/CIRCULATIONAHA.107.742510. PMID 18506008.
- ↑ "Drug Approval Package: Letairis (ambrisentan) NDA #022081". 24 December 1999. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022081s000TOC.cfm.
- ↑ "Ambrisentan Orphan Drug Designation and Approval". https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=183604.
- ↑ "Gilead's Drug Is Approved to Treat a Rare Disease". The New York Times. 16 June 2007. https://www.nytimes.com/2007/06/16/business/16gilead.html.
- ↑ "Endothelin-1 receptor antagonists in fetal development and pulmonary arterial hypertension". Reproductive Toxicology 56: 45–51. 2015. doi:10.1016/j.reprotox.2015.06.048. PMID 26111581.
- ↑ "Initial Use of Ambrisentan plus Tadalafil in Pulmonary Arterial Hypertension". The New England Journal of Medicine 373 (9): 834–44. August 2015. doi:10.1056/NEJMoa1413687. PMID 26308684.
 |

