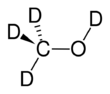
Chemistry:Deuterated methanol
From HandWiki
Short description: Chemical compound

| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
(2H3)Methan(2H)ol | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 1733278 | |||
| ChEBI | |||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| UN number | 1230 | ||
| |||
| |||
| Properties | |||
| CD4O | |||
| Molar mass | 36.0665 g mol−1 | ||
| Density | 0.888 g cm−3 | ||
| Melting point | −98 °C (−144 °F; 175 K) | ||
| Boiling point | 65 °C (149 °F; 338 K) | ||
| Thermochemistry | |||
Heat capacity (C)
|
87.9 J K−1 mol−1 | ||
| Hazards | |||
| GHS pictograms |   
| ||
| GHS Signal word | Warning | ||
| H225, H301, H311, H331, H370 | |||
| P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P280, P301+310, P302+352, P303+361+353, P304+340, P307+311, P311, P312, P321, P322, P330, P361, P363, P370+378 | |||
| Flash point | 11 °C (52 °F; 284 K) | ||
| Related compounds | |||
Related compounds
|
Methanol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Tracking categories (test):
Deuterated methanol (CD3OD), is a form (called an isotopologue) of methanol (CH3OH) in which the hydrogen atoms ("H") are replaced with deuterium (heavy hydrogen) isotope ("D").[1] Deuterated methanol is a common solvent used in NMR spectroscopy.
Deuterated methanol was first detected in interstellar space was Orion-KL in 1988 by scientists at the Max Planck Institute for Radio Astronomy.[2]
References
- ↑ Bizzocchi, L.; Caselli, P.; Spezzano, S.; Leonardo, E. (2014-09-01). "Deuterated methanol in the pre-stellar core L1544" (in en). Astronomy & Astrophysics 569: A27. doi:10.1051/0004-6361/201423858. ISSN 0004-6361. Bibcode: 2014A&A...569A..27B. https://www.aanda.org/articles/aa/abs/2014/09/aa23858-14/aa23858-14.html.
- ↑ Mauersberger, R.; Henkel, C.; Jacq, T.; Walmsley, C. M. (1988-04-01). "Deuterated methanol in Orion.". Astronomy and Astrophysics 194: L1–L4. ISSN 0004-6361. Bibcode: 1988A&A...194L...1M. https://ui.adsabs.harvard.edu/abs/1988A&A...194L...1M.
 |