Chemistry:Devimistat
 | |
| Clinical data | |
|---|---|
| Other names | CPI-613 |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
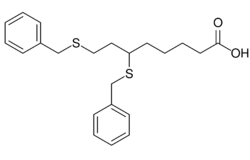
| Formula | C22H28O2S2 |
| Molar mass | 388.58 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Devimistat (INN; development code CPI-613) is an experimental anti-mitochondrial drug being developed by Cornerstone Pharmaceuticals.[1] It is being studied for the treatment of patients with metastatic pancreatic cancer and relapsed or refractory acute myeloid leukemia (AML).
Devimistat's mechanism of action differs from other drugs, operating on the tricarboxylic acid cycle and inhibiting enzymes involved with cancer cell energy metabolism. A lipoic acid derivative different from standard cytotoxic chemotherapy, devimistat is currently being studied in combination with modified FOLFIRINOX to treat various solid tumors and heme malignancies.
Regulation
The U.S. Food and Drug Administration (FDA) has designated devimistat as an orphan drug for the treatment of pancreatic cancer, AML, myelodysplastic syndromes (MDS), peripheral T-cell lymphoma, and Burkitt's lymphoma, and given approval to initiate clinical trials in pancreatic cancer and AML.[citation needed]
Clinical trials
Clinical trials of the drug are underway including a Phase III open-label clinical trial[2] to evaluate efficacy and safety of devimistat plus modified FOLFIRINOX (mFFX) versus FOLFIRINOX (FFX) in patients with metastatic adenocarcinoma of the pancreas.[citation needed]
References
- ↑ "CPI-613". Cornerstone Pharmaceuticals. https://cornerstonepharma.com/research-and-development/cpi-613-drug.
- ↑ "A Phase III open-label trial to evaluate efficacy and safety of CPI-613 plus modified FOLFIRINOX (mFFX) versus FOLFIRINOX (FFX) in patients with metastatic adenocarcinoma of the pancreas". Future Oncology 15 (28): 3189–3196. October 2019. doi:10.2217/fon-2019-0209. PMID 31512497.
 |
