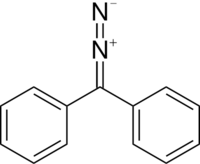
Chemistry:Diazodiphenylmethane
| |
| Names | |
|---|---|
| Preferred IUPAC name
(Diazomethylene)dibenzene | |
| Other names
Diazodiphenylmethane
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| MeSH | C480088 |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C13H10N2 | |
| Molar mass | 194.237 g·mol−1 |
| Appearance | red-black solid |
| Melting point | 30 °C (86 °F; 303 K) |
| −115·10−6 cm3·mol−1 | |
| Hazards | |
| Main hazards | unstable |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Diazodiphenylmethane is an organic reagent with the chemical formula C13H10N2. It exists as red-black crystals that melts just above room temperature.[1]
Preparation
Diazodiphenylmethane can be synthesized via the oxidation of benzophenone hydrazone with mercury(II) oxide in diethyl ether and the presence of a basic catalyst.[2] An improved procedure involves dehydrogenation with oxalyl chloride.[3]
Uses
It can be used to synthesise (diphenyl)methyl esters and ethers with carboxylic acids and alcohols respectively.[4][5]
It can also generate the (diphenyl)methyl carbene and nitrogen gas upon illumination by ultraviolet light or heating.[6][7] It can also be electrolysed to form the Ph2CN−2 anion, which can decompose to form the Ph2C− anion radical. If carried out in dimethylformamide and tetrabutylammonium perchlorate, these can react to form benzophenone azine, which has the formula Ph2C=N-N=CPh2.[8]
References
- ↑ "Diphenyldiazomethane;1,1'-(Diazomethylene)bisbenzene;Diazodiphenylmethane,physical properties,suppliers,CAS,MSDS,structure,Molecular Formula, Molecular Weight ,Solubility,boiling point, melting point". Archived from the original on 2016-08-04. https://web.archive.org/web/20160804033036/http://chemyq.com/En/xz/xz1/8364rohuk.htm.
- ↑ Miller, J (1959-04-01). "Notes- Preparation of Crystalline Diphenyldiazomethane". The Journal of Organic Chemistry 24 (4): 560–561. doi:10.1021/jo01086a603. ISSN 0022-3263.
- ↑ "orgsyn.org/demo.aspx?prep=V85P0189". http://orgsyn.org/demo.aspx?prep=V85P0189.
- ↑ Jovanovic, Bratislav; Assaleh, Fathi; Marinkovic, Aleksandar (2004). "Kinetics of the reaction of 5-substituted orotic acids with diazodiphenylmethane". Journal of the Serbian Chemical Society 69 (11): 949–953. doi:10.2298/jsc0411949j. http://www.doiserbia.nb.rs/Article.aspx?ID=0352-51390411949J.
- ↑ Petursson, Sigthor (2003-04-22). "Tin(II) chloride catalyzed reactions of diazodiphenylmethane with vicinal diols in an aprotic solvent. The reactions with cis- and trans-1,2-cyclohexanediols and 1,2-propanediol". Carbohydrate Research 338 (9): 963–968. doi:10.1016/S0008-6215(03)00039-9.
- ↑ Parker, Vernon D.; Bethell, Donald (1987-08-01). "Carbene cation radicals: the kinetics of their formation from diazoalkane cation radicals and their reactions". Journal of the American Chemical Society 109 (17): 5066–5072. doi:10.1021/ja00251a002. ISSN 0002-7863.
- ↑ Sabongi, Gebran J. (2012-12-06) (in en). Chemical Triggering: Reactions of Potential Utility in Industrial Processes. Springer Science & Business Media. ISBN 9781461309079. https://books.google.com/books?id=FEPaBwAAQBAJ.
- ↑ McDonald, Richard N.; Triebe, F. M.; January, J. R.; Borhani, K. J.; Hawley, M. D. (1980-12-01). "Hypovalent radicals. 6. Electroreduction of diazodiphenylmethane - intermediacy of Ph2CN2-.cntdot. and Ph2C-.cntdot.". Journal of the American Chemical Society 102 (27): 7867–7872. doi:10.1021/ja00547a007. ISSN 0002-7863.
 |

